All published articles of this journal are available on ScienceDirect.
Efficacy and Safety of Modified Pranlukast (Prakanon®) Compared with Pranlukast (Onon®): A Randomized, Open-Label, Crossover Study
Abstract
Introduction:
Pranlukast is a leukotriene receptor antagonist (LTRA) that is used as an additional controller of mild to moderate asthma. This study compared the efficacy and side effects of two bioequivalent preparations of pranlukast: original pranlukast (Onon®; Ono Pharmaceutical, Japan) and a modified formulation of pranlukast (Prakanon®; Yuhan Co, Korea) in patients with mild to moderate asthma.
Methods:
Of the 34 subjects screened, 30 patients who were using standard medication to control asthma and scored less than 20 points on the Asthma Control Test™ (ACT) were assigned randomly to one of the two groups in a prospective, open label, crossover study: group 1 received Prakanon® (150 mg/day) and group 2 received Onon® (450 mg/day) for 8 weeks each; after a 1-week rest period, the groups were switched to the alternative medication for further 8 weeks and monitored for 2 more weeks without study medication. Evaluation parameters included the ACT, quality of life questionnaire adult Korean asthmatics (QLQAKA), pulmonary function tests, peripheral blood tests, vital signs, and adverse events.
Results:
Thirty patients were enrolled and 21 completed the trial: 10 in group 1 and 11 in group 2. The baseline data of the two groups did not differ. No statistical significant differences were observed in efficacy and lung function at each time and in changes from baseline value between the two kinds of pranlukast. The final asthma control rate was 81% with Prakanon® and 76% with Onon®. There were no differences in vital signs and laboratory data at each time and in changes from baseline value between the two drugs. There were no differences in adverse events between the two drugs. The most common side effect was abdominal pain. Drug compliance was high, without differences between the two drugs.
Conclusion:
These findings suggest that Prakanon® which is an improved formulation of pranlukast at a lower dose than the original formulation, Onon®, has a similar efficacy and side effect profile in the control of persistent asthma.
INTRODUCTION
Asthma is a chronic inflammatory allergic disease of the airway in which various cells and media are involved [1]. Inflammation of the airway causes symptoms such as recurrent wheezing and difficulty in breathing, cough, chest pain, and combined nasal symptoms related to airway allergic irritation [2]. Although the complex mechanism of asthma has not been clearly elucidated, it is known that various allergic inflammatory cells, including T lymphocytes, B lymphocytes, eosinophils, and mast cells, as well as cytokines and chemokines that control the chemical media, are involved in the pathology [3]. Drugs that are currently in use for asthma management promote symptom relief by controlling the inflammatory processes of the airway.
Leukotriene, an important inflammatory mediator for airway inflammation of asthma, causes the contraction of airway smooth muscle, mucus secretion, increased vascular permeability, and inflammatory cell infiltration [4, 5]. Leukotriene receptor antagonists (LTRAs) can improve the pulmonary function of asthmatics and reduce airway inflammation, the number of asthma attacks, and the use of short acting inhaled bronchodilators. LTRAs can be used not only as an additional therapeutic agents for mild to moderate asthma or to protect from severe asthmatic attack, but also as an option in noncompliant subjects to inhaled agents [6-10].
Pranlukast is a drug with proven effectiveness and stability, which is used as a therapeutic agent for bronchial asthma after being developed as a selective LTC4/D4/E4 receptor blocker in Japan, 1995 [11, 12]. The effect of pranlukast has also been proven for mild to moderate asthma in Korea, 2001 [13].
Yuhan Cooperation recently developed a modified formulation of pranlukast, which has a profile that shows equal bioavailability in vitro at lower concentrations compared to the original formulation [14]. However, to date no reports have investigated the bioequivalence in human subjects. Hence, this study compared the effectiveness and adverse reactions of Prakanon® (150 mg/day) and Onon® (450 mg/day) in mild and moderate persistent asthma.
METHODS
This clinical trial adopted a phase 4, randomized, open label, cross-over design as a pilot study based at a single institute to evaluate the efficacy and safety of the modified formulation of pranlukast hydrate 150 mg (Prakanon®) compared to the original pranlukast hydrate 450 mg (Onon®) in the treatment of mild to moderate persistent asthmatics. It began in June 2011 and was completed in March 2013 at Ewha Womans University Mokdong Hospital. This study was approved by the Institutional Review Board of Mokdong Hospital (2011-0014-1-1) and registered in the WHO International Clinical Trials Registry (KCT0000457).
Thirty-four subjects were screened as a pre-targeted sample number and 30 subjects were assessed as eligible and randomized for this trial. The statistician made a random number table to divide the enrolled patients into two groups and the numbers were allocated by the study coordinator without notifying the principal investigator. Intent-to-treat (ITT) population for analysis was defined to subjects who had undertaken more than 1 time evaluation since baseline examination and retrieved data from them were used for the evaluation of efficacies.
Subjects were enrolled for uncontrolled asthma according to following criteria. First, the subjects were persistent asthmatic on step 2 or 3 of GINA guideline [15] using beclomethasone dipropionate (CFC) more than 200µg per day or inhaled corticosteroid (ICS) of similar biologic equivalent potency. Second, the subject presented less than 20 points on the Asthma Control Test™ (ACT) [16] due to unsuccessful asthma control at the time of registration even though the subject had been treated for more than one month with usual asthma care. Subjects were excluded if they smoked more than 20 packs-year, suffered lung infiltration visualized by chest x-ray that could affect pulmonary function, used a leukotriene modifier within 4 weeks from the time of registration, used systemic corticosteroids or immunosuppressive drugs, had congestive heart failure, chronic liver disease, or chronic renal disease, or had been diagnosed with a malignant tumor within the past 5 years. All subjects continued their previous medication through out the study. Group 1 was given Prakanon® 150 mg per day (75 mg, bid) for 8 weeks, 1 week drug free interval, followed by 8 weeks of Onon® 450 mg per day (225 mg, bid). Group 2 was given Onon® 450 mg per day (225 mg, bid) for 8 weeks, 1 week free interval, followed by 8 weeks of Prakanon® 150 mg per day (75 mg, bid). Both groups were monitored for 2 more weeks after 17 weeks’ progress without study medication (Fig. 1).
The primary outcome of this study involved comparison between Prakanon® and Onon® in the differences of ACT score at 8 weeks from baseline during the trial. Secondary outcomes included comparison between Prakanon® and Onon® in the differences of ACT score at 4 weeks from baseline and in the differences of the quality of life questionnaire adult Korean asthmatics (QLQAKA) score each at 4 weeks and 8 weeks from baseline [17]. Pulmonary functional parameters, vital signs, and basic hematological parameters during the trial were analyzed similarly. Adverse events and drug compliances were monitered during the trial.
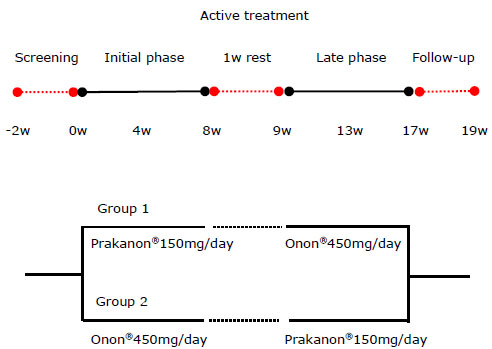
Study design of 19 week(w)s' trial.
Statistical Evaluation
The two-sample Student’s t-test was used to compare the two groups in terms of the effects of treatment, the effect of period, and the carryover effect for the cross-over design. Two-sample Student’s t-test or Wilcoxon’s rank sum test was used to compare the means from the two different groups. Regarding the incidence of adverse events observed after administration of the test drug, Fisher’s exact test or the chi-square test was used to assess significance in both groups. Mixed effect model with sequence, period, and treatment as fixed effects and subject within the sequence as a random effect was employed using the PROC MIXED SAS procedure to compare compliance rates of the two drugs. All statistical analyses were conducted using the statistical software SAS system 9.1.3 (SAS Institute, Cary, NC, USA) and p < 0.05 was considered to indicate statistical significance.
RESULTS
Of the 30 subjects, 14 subjects were allocated to group 1 and 16 subjects to group 2. In group 1, 4 subjects withdrew, 3 subjects withdrew consent, 1 subject experienced adverse events, and 10 subjects completed the trial. In group 2, 5 subjects withdrew, 2 subjects withdrew consent, 2 subjects had loss of follow-up, 1 experienced adverse events and finally 11 subjects completed the trial. The number of ITT population according to the definition was 21 (Fig. 2).
The two groups did not differ in patient information including age, sex, body mass index, smoking history, duration of asthma, number of asthma medications, recent history of emergency medication, combined illnesses, the ACT, the QLQAKA, and the pulmonary function parameter at baseline (Table 1). On scheduled treatment with the two drugs, the scores of ACT and QLQAKA were improved, but the differences in each group at 4th and 8th weeks from baseline levels (post-treatment minus baseline) in ACT and QLQAKA were not significant between Prakanon® and Onon®. The changes in pulmonary function parameters of FEV1, FEV1/FVC, and PEF each at 4th and 8th week from baseline levels were not significant between the two drugs (Table 2).
Demographic data of intent-to-treat (ITT) population at baseline.
| Group 1 | Group 2 | p value | |
|---|---|---|---|
| Number | 10 | 11 | |
| Age, year | 44 ± 12 | 49 ± 13 | NSa |
| Sex: male | 8 (80) | 7 (64) | NSb |
| Body Mass Index, kg/m2 | 25.5 ± 3.3 | 25.0 ± 4.7 | NSa |
| Smoker | 8 (80) | 7 (64) | NSb |
| Duration of asthma | NSb | ||
| less than 1 year | 5 (50) | 2 (18.2) | |
| 1 year - 3 years | 2 (20) | 4 (36.4) | |
| 3 years - 5 years | 1 (10) | 1 (9) | |
| more than 5 years | 2 (20) | 4 (36.4) | |
| Number of asthma medication | NSb | ||
| one | 9 (90) | 11 (100) | |
| 2 and more | 1 (10) | 0 (0) | |
| Recent history of emergency medication | 2 (20) | 3 (27) | NSb |
| Combined illness | |||
| Diabetes | 1 (10) | 0 (0) | NSb |
| Hypertension | 0 (0) | 1 (9) | NSb |
| Rhinitis | 4 (40) | 1 (9) | NSb |
| Gastritis | 2 (20) | 1 (9) | NSb |
| Cerebrovascular disease | 0 (0) | 1 (9) | NSb |
| Asthma status | |||
| ACT score | 16.3 ± 2.6, | 15.8 ± 3.8 | NSa |
| QLQAKA score | 63.2 ± 9.5 | 64.7 ± 13.9 | NSa |
| FEV1(ℓ) | 3.1 ± 1.0 | 2.7 ± 0.8 | NSa |
| FEV1, % predicted | 89 ± 16 | 85 ± 14 | NSa |
| FEV1/FVC (%) | 77.9 ± 12.2 | 68.8 ± 8.4 | NSa |
| PEF (ℓ/min) | 7.6 ± 2.6 | 6.5 ± 2.1 | NSa |
Efficacy evaluation of ITT population according to the treatment sequences.
| Prakanon® (n=21a) | Onon® (n=21) | p value | |
|---|---|---|---|
| ACT | |||
| Baseline (week 0, week 9) | 18.2 ± 4.1 | 18.2 ± 4.3 | NS |
| 4th week (week 4, week 13) | 20.6 ± 4.4 | 20.7 ± 4.3 | NS |
| Changes from baseline | 2.3 ± 4.3 | 2.5 ± 3.9 | NSb |
| 8th week (week 8, week 17) | 20.9 ± 3.2 | 21.3 ± 3.3 | NS |
| Changes from baseline | 2.7 ± 3.2 | 3.1 ± 4.2 | NSc |
| QLQAKA | |||
| Baseline (week 0, week 9) | 67.8 ± 11.5 | 68.9 ± 12.2 | NS |
| 4th week (week 4, week 13) | 71.7 ± 13.7 | 73.0 ± 11.7 | NS |
| Changes from baseline | 3.9 ± 10.9 | 4.1 ± 9.6 | NSb |
| 8th week (week 8, week 17) | 73.4 ± 11.1 | 72.9 ± 11.7 | NS |
| Changes from baseline | 5.6 ± 10.0 | 4.0 ± 11.1 | NSc |
| FEV1 | |||
| Baseline (ℓ) (week 0, week 9) | 2.9 ± 0.9 | 2.9 ± 0.9 | NS |
| FEV1, % predicted | 85 ± 15 | 88 ± 15 | NS |
| 4th week (ℓ) (week 4, week 13) | 3.0 ± 0.9 | 3.0 ± 0.9 | NS |
| Changes from baseline | 0.11 ± 0.37 | 0.05 ± 0.35 | NSb |
| FEV1, % predicted | 89 ± 14 | 84 ± 24 | NS |
| 8th week (ℓ) (week 8, week 17) | 3.1 ± 1.2 | 3.0 ± 0.9 | NS |
| Changes from baseline | 0.25 ± 0.74 | 0.04 ± 0.29 | NSc |
| FEV1, % predicted | 87 ± 14 | 87 ± 13 | NS |
| FEV1/FVC (%) | |||
| Baseline (week 0, week 9) | 72.3 ± 11.2 | 73.4 ± 10.6 | NS |
| 4th week (week 4, week 13) | 72.9 ± 12.2 | 72.7 ± 9.4 | NS |
| Changes from baseline | 0.57 ± 9.05 | -0.01 ± 6.84 | NSb |
| 8th week (week 8, week 17) | 76.7 ± 15.6 | 73.5 ± 9.8 | NS |
| Changes from baseline | 4.38 ± 13.52 | 0.09 ± 4.89 | NSc |
| PEF (ℓ/min) | |||
| Baseline (week 0, week 9) | 7.0 ± 2.3 | 7.1 ± 2.4 | NS |
| 4th week (week 4, week 13) | 7.4 ± 2.5 | 7.6 ± 2.5 | NS |
| Changes from baseline | 0.42 ± 1.23 | 0.27 ± 1.17 | NSb |
| 8th week (week 8, week 17) | 7.5 ± 2.7 | 7.2 ± 2.6 | NS |
| Changes from baseline | 0.54 ± 1.04 | 0.06 ± 0.97 | NSc |
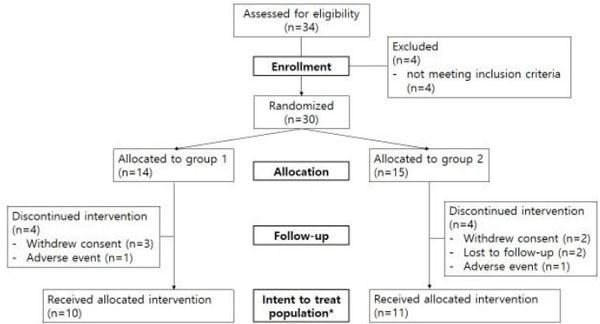
The Consort flowchart.
*ITT population was defined as subjects who were monitored more than 1 time since baseline visit.
Asthma control in Prakanon® use in group 1 was achieved in 7 out of 10 subjects (Fig. 3a, solid line) and Onon® achieved asthma control in 7 out of 10 subjects (Fig. 3b, solid line). In group 2, asthma control after using Onon® was achieved in 9 out of 11 subjects (Fig. 3c, solid line) and following Prakanon® achieved asthma control in 10 out of 11 subjects (Fig. 3d, solid line). The final asthma control rate was 17/21 (81%) with Prakanon® and 16/21 (76%) with Onon®. Clinical failure on the effect of LTRA was observed in 5 (24%) of the 21 subjects who completed the trial during the initial 8 weeks of treatment (Fig. 3a & 3c, dotted line). In the later 9th week to 17th week of the trial, after the crossover, most subjects maintained previous response to the ACT, except in 4 cases (19%) of clinical failure (Fig. 3b & 3d, dotted line).

Outcome of ACT according to the time course with additional LTRA.
*The solid line shows the clinical achievement of asthma control with LTRA treatment and the dotted line indicates uncontrolled status of asthma at the end of therapy.
Among the 30 registered subjects, the vital signs and basic blood examinations were compared before and after the clinical trial and there were no differences at baseline and 8th week, and no differences in changes from baseline levels between the two drugs (Table 3).
Vital signs and laboratory data, monitored during study.
| Prakanon® (n=30a) | Onon® (n=30) | p value | |
|---|---|---|---|
| Systolic BP | |||
| Baseline (week 0, week 9) | 127 ± 11 | 129 ± 12 | NS |
| 8th week (week 8, week 17) | 122 ± 13 | 127 ± 14 | NS |
| Changes from baseline | -5.29 ± 10.96 | -1.55 ± 13.6 | NSb |
| Diastolic BP | |||
| Baseline (week 0, week 9) | 80 ± 8 | 77 ± 12 | NS |
| 8th week (week 8, week 17) | 77 ± 10 | 75 ± 11 | NS |
| Changes from baseline | -2.95 ± 8.32 | -0.91 ± 12.74 | NSb |
| Pulse rate | |||
| Baseline (week 0, week 9) | 81 ± 8 | 82 ± 9 | NS |
| 8th week (week 8, week 17) | 80 ± 9 | 80 ± 14 | NS |
| Changes from baseline | -1.05 ± 8.62 | -3.05 ± 15.75 | NSb |
| WBC/mm3 | |||
| Baseline (week 0, week 9) | 8270 ± 1930 | 7160 ± 1630 | NS |
| 8th week (week 8, week 17) | 6550 ± 1500 | 6700 ± 1940 | NS |
| Changes from baseline | -1140 ± 1220 | -170 ± 1170 | NSb |
| Eosinophils (%) | |||
| Baseline (week 0, week 9) | 3.4 ± 1.9 | 4.9 ± 4.4 | NS |
| 8th week (week 8, week 17) | 3.9 ± 3.1 | 4.1 ± 3.0 | NS |
| Changes from baseline | -1.51 ± 0.88 | -0.04 ± 2.46 | NSb |
Adverse events did not differ between the use of Prakanon® and Onon®. The adverse events that caused discontinuation of the drug, regardless of the direct relationship with the study drug, were severe itching and sudden mitral valve dysfunction, which occurred during the use of Prakanon®. An acute exacerbation of asthma was occurred in one subject during Prakanon® treatment. During the clinical trial, the most common drug-related side effects were stomachache, diarrhea, pruritus, and abnormal liver function (Table 4). Drug compliance was high for both groups and did not differ between the two drugs each at 4th week and 8th week (Table 5).
Adverse events monitored during the study.
| Prakanon® (n=30a) | Onon® (n=30) | p-valueb | |
|---|---|---|---|
| Adverse events (AEs) | 11(33%) | 11(33%) | NS |
| Drug related AEs | 5 | 5 | NS |
| Abdominal pain | 1 | 3 | |
| Diarrhea | 1 | 1 | |
| Itching | 2 | 0 | |
| Urticaria | 0 | 1 | |
| Elevation of ALT and bilirubin | 1 | 0 | |
| Non drug related AEs | 6 | 6 | NS |
| Flu-like illness | 5 | 4 | |
| Hemorrhoid | 0 | 1 | |
| Transient muscle ache | 0 | 1 | |
| Rheumatic heart failure | 1 | 0 | |
| Serious AE | 1 | 0 | NS |
| AE leading to drug discontinuation | 2 | 0 | NS |
Drug compliance at 4th week and 8th week using Prakanon® and Onon®.
| Prakanon® (n=30a) | Onon® (n=30) | p-valueb | |
|---|---|---|---|
| Compliance (%, 4th week) | 92.0 ± 14.0 | 90.8 ± 13.3 | 0.86 |
| Compliance (%, 8th week) | 94.8 ± 6.4 | 96.0 ± 7.0 | 0.58 |
DISCUSSION
These findings indicate that Prakanon®, the new formulation of pranlukast has a similar biologic efficacy in vivo even with a 1/3 dosage of the original formulation, Onon®.
The goal of asthma treatment is to allow the patient to lead a normal life as a non-asthmatic person. The stepwise approach according to the severity of asthma, presented by a patient was one of the most important processes in asthma management. From step 1 to step 5, inhaled corticosteroid is baseline standard medication. With the elevation of severity, theophylline, LTRA, long acting beta agonist (LABA), and long acting muscarinic agonist (LAMA) are added sequentially or in combination as anti-inflammatory bronchodilator [15]. Recently, complete control of asthma has the following issues: absence of symptoms, less use of relievers, and absence of experiencing exacerbation. Contrary to that, uncontrolled asthma exhibits persistent symptoms and exacerbations despite current asthma management [15]. For effective control of asthma, physicians prescribe a combination inhaler containing ICS and LABA or add other medications such as LTRAs, when not controlled with ICS [18].
LTRAs work by blocking a chemical reaction that can lead to inflammation in the airways. LTRAs have a role as an additional therapy for ICS or ICS/LABA combination therapy for the control of asthma in terms of pulmonary functions and biologic markers as well as symptoms [11, 13, 19]. Especially for a step-up treatment, LTRA is recommended in steps 2, 3, and 4 of asthma [15]. Because high dose of ICS has a safety issue related to the risk of infection and has limited effects on control, it is primarily offered to add an LTRA or LABA rather than increasing ICS [18]. LTRA is indicated as a substitute when an inhaled steroid cannot be used, or if the dose cannot be increased [20]. Also, it is useful for the prevention of exercise induced bronchoconstriction [21].
The ACT is the most universal objective numerical index providing the status of asthma control over the recent 4 weeks [16, 22]. Full score of 25 points on 5 categories of 5 point scale means complete control; 20 points and more indicate well control; less than 20 points reflect poor control [16]. The QLQAKA is an index of quality of life, developed from Korea with higher scores representing a better quality of life [17]. This study involved mild to moderate asthmatics in uncontrolled status. The subjects at the time of enrollment had initial baseline ACT scores of less than 20 points even with the maintenance of their usual asthma medication and they were given an additional LTRA. Each treatment of Prakanon® and Onon® for 8 weeks confirmed that both therapies yielded an improved status of ACT at 4th week and this status had been maintained until 8 weeks within an acceptable range. Also, QLQAKA scores were slightly improved at the 4th and 8th week of each treatment, without differences between two drugs. Yasui et al. [23] reported that both the morning peak expiratory flow (PEF) and ACT improved after additional use of pranlukast, among the moderate to severe asthma patients who used ICS. The present study suggests that pranlukast is effective in treating uncontrolled asthma especially with the use of ICS, and there is no difference in the effectiveness of the two different formulations of the same active compound. Although no statistically significant differences were observed in the comparison before and after treatment regardless of drug formulations according to the pulmonary function index measured in this research, the average values after the intervention were similar or had increased compared to the levels before treatment.
Research has found no significant side effects associated with Prakanon®, compared to Onon®, which were monitored for 19 weeks. According to a recent report, the concentration of LTRA increased in liver, and caused headache and nausea [24]. Obase et al. [25] reported side effects of pranlukast including diarrhea, dizziness, and bilateral leg edema within the first 4 weeks of starting the medication. In this study, abdominal pain and diarrhea were the main side effects related to pranlukast. The itching in the Prakanon® has not been previously reported.
The study has the following limitations: a study conducted in one hospital, small sample size, and relatively short observational period to evaluate side effects. However, this study verified that Prakanon®, the new formulation of pranlukast, has useful pharmacologic efficacy and tolerable adverse effects.
Overall, Prakanon® was as effective as Onon® for the treatment of uncontrolled asthma and the adverse effects of the two drugs were comparable.
CONCLUSION
This phase 4 clinical trial suggests that Prakanon®, an improved new drug formulation with biological similarity, but at a lower dose, compared to Onon®, has similar effectiveness and side effects in the treatment of mild to moderate asthma. Large long-term scale studies are required to clarify these results.
LIST OF ABBREVIATIONS
| ACT | = Asthma Control Test™ |
| ICS | = Inhaled corticosteroid |
| LABA | = Long-acting beta 2-agonist |
| LTRAs | = Leukotriene receptor antagonists |
| PEF | = Peak expiratory flow |
| QLQAKA | = Quality of life questionnaire adult Korean asthmatics |
| WBCs | = White blood cells |
CONFLICT OF INTEREST
This study was funded by Yuhan Corporation (YMC 003, version 6). The role of funding company was limited to the study design and data analysis. The funding company was not involved in conducting the study, data interpretation, or manuscript preparation. None of the authors have any relevant conflicts of interest.
ACKNOWLEDGEMENTS
The role of corresponding author was to provide the study design to conduct the study, analysis, interpretation of data, and preparation of the manuscript. All authors contributed to data collection and writing substantially.


