All published articles of this journal are available on ScienceDirect.
Pilot Study of a New Adjustable Thermoplastic Mandibular Advancement Device for the Management of Obstructive Sleep Apnoea-Hypopnoea Syndrome: A Brief Research Letter
Abstract
Background:
Prefabricated adjustable thermoplastic mandibular advancement devices (PAT-MADs) are a practical short-term treatment for obstructive sleep apnoea-hypopnoea syndrome (OSAHS) in patients who have failed or refused continuous positive airway pressure (CPAP) therapy.
Objective:
To assess the effectiveness of a new professionally-fitted PAT-MAD in patients with OSAHS in Morocco.
Method:
Twenty-four adults with mild, moderate or severe OSAHS were fitted with the PAT-MAD (BluePro®; BlueSom, France). Respiratory parameters (apnoea-hypopnoea index (AHI), oxygen desaturation index (ODI)) and daytime sleepiness using the Epworth Sleepiness scale (ESS) were assessed before and after treatment. Adverse events were recorded.
Results:
Mean treatment duration was 106.3 ± 73.4 days. Mean AHI score decreased from 21.4 ± 7.4 to 9.3 ± 4.1 after treatment (p<0.0001) (mean reduction of 57.0 ± 12.3%). Mean ESS and ODI also decreased at EOS (from 10.4 ± 2.8 to 7.3 ± 2.3, mean reduction 30.3 ± 12.2%, p=0.0001; and 7.0 ± 6.9 to 4.7 ± 4.0, mean reduction 30.5 ± 25.0%, p=0.2, respectively). Treatment was considered to have been successful in 22 patients (91.7%) who had mild OSAHS or an AHI score of ≤5 at the end of the study. The device was well-tolerated.
Conclusion:
This new PAT-MAD appears to be effective at reducing respiratory parameters and improving daytime alertness in patients with OSAHS. Long term studies in a larger number of patients are warranted to assess the long-term efficacy, retention and side-effects of this device.
INTRODUCTION
Continuous positive airway pressure (CPAP) is the gold standard treatment for moderate to severe obstructive sleep apnoea-hypopnoea syndrome (OSAHS) [1-3]. Maintenance of upper airway patency during sleep can also be achieved using a mandibular advancement device (MAD). Prefabricated adjustable thermoplastic MADs (PAT-MADs) are a practical short-term treatment for OSAHS in patients who have failed or refused CPAP therapy [4-7]. Custom-made MADs have been shown to achieve higher rates of improvement and OSAHS cure than prefabricated MADs due to compliance failure with prefabricated devices, mainly caused by insufficient overnight retention [8, 9]. However custom-made devices have the disadvantage of higher cost [8] and a delay in treatment while the device is manufactured.
Our purpose was to describe the changes in respiratory parameters (apnoea-hypopnoea index (AHI), oxygen desaturation index (ODI)) and Epworth Sleepiness Scale (ESS) in Moroccan patients with OSAHS after treatment with a new retentive PAT-MAD.
MATERIALS AND METHODS
Twenty-four adults (20 male; mean age 48.6 ± 9.3 years) with mild, moderate or severe OSAHS diagnosed by polysomnography (PSG) and poor compliance or rejection of CPAP were enrolled. Exclusion criteria included: dental syndrome (i.e. propulsion <6 mm), dental/peridontal disease/caries, heavily restored dentition, insufficient number of teeth, temporomandibular joint (TMJ) disorder, significant tooth wear, non-retentive teeth, poor oral hygiene or inadequate mouth opening. As this was a prospective, observational, pilot study a sample size calculation was not performed and we considered that the recruitment of 20 or more patients would give reliable information. The study was approved by the local ethics committee and all patients gave their written informed consent.
Each patient was fitted with the PAT-MAD (BluePro®; BlueSom, France; Fig. (1) by their dental practitioner Subsequent PAT-MAD adjustments were made by the patient according to the manufacturer’s instructions until snoring ceased or decreased and there was maximum comfortable advancement.
BluePro® prefabricated adjustable thermoplastic mandibular advancement device.
AHI score [10] for each patient was measured by PSG at inclusion and at the end of the study (EOS). Severity of OSAHS was defined by AHI score as: mild=AHI <15; moderate=AHI 15–29; or severe=AHI ≥30. Successful treatment was defined as a ≥50% reduction in AHI to mild OSAHS, or a reduction in AHI to ≤5/h. ODI score [11] was also measured at inclusion and EOS by nocturnal oximetry and daytime sleepiness was measured using a patient self-questionnaire and the ESS scale [12].
Five items relating to the efficacy and acceptance of the device (snoring, comfort, TMJ pain, dental pain, satisfaction; each on a scale of 0-10) and five items relating to device use (retention, frequency of use (h/night and nights/week), number of advancements made and acceptance of the device long-term) were also recorded at EOS using a patient self-questionnaire. All adverse events (AEs) that occurred during use of the PAT-MAD were recorded.
RESULTS
The demographic and clinical characteristics of the study population at inclusion are shown in Table 1.
Demographic and clinical characteristics of the study population fitted with the prefabricated adjustable thermoplastic mandibular advancement device (PAT-MAD).
| Characteristic | (N=24) |
|---|---|
| Sex (male) | 20 (83.3%) |
| Age (years)* | 48.6 ± 9.3 |
| BMI (kg/m2) | 26.0 ± 2.5 |
| Previous treatment | |
| Other MAD | 18 (75%) |
| CPAP | 6 (25%) |
| Severity of OSAHS at inclusion | |
| Mild | 6 (25%) |
| Moderate | 15 (62.5%) |
| Severe | 3 (12.5%) |
| Score at inclusion before using the PAT-MAD | |
| AHI | 21.4 ± 7.4 |
| ESS | 10.4 ± 2.8 |
| ODI | 7.0 ± 6.9 |
| Follow-up (days) | 106.3 ± 73.4 |
| Use (days/week) | 5.6 ± 1.7 |
| Use (h/night) | 5.4 ± 1.5 |
| Number of mandibular advancements made | 2.0 ± 1.0 |
| Score at EOS after using the PAT-MAD | |
| AHI | 9.3 ± 4.1 |
| ESS | 7.3 ± 2.3 |
| ODI | 4.7 ± 4.0 |
The new PAT-MAD was effective at reducing respiratory parameters in patients with OSAHS, including three with severe disease, when used for a mean time of 106.3 ± 73.4 days, 5.6 ± 1.7 nights/week and 5.4 ± 1.5 h/night. Mean AHI decreased significantly between inclusion and EOS (from 21.4 ± 7.4 to 9.3 ± 4.1; p<0.0001; mean reduction: 57.0 ± 12.3%) Fig. (2A). Mean ESS also decreased significantly at EOS (from 10.4 ± 2.8 to 7.3 ± 2.3; p=0.0001; mean reduction: 30.3 ± 12.2%) Fig. (2B). A non-significant reduction in mean ODI was observed at EOS (from 7.0 ± 6.9 to 4.7 ± 4.0%; p=0.2; mean reduction: 30.5 ± 25.0%) Fig. (2C). Treatment was considered to have been successful in 22 patients (91.7%) who had mild OSAHS or an AHI score of ≤5 at EOS. A mean of 2 ± 1 advancements were made to the PAT-MAD during treatment.
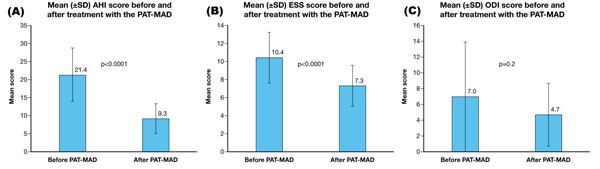
Mean scores (± SD) before and after treatment with the PAT-MAD.
The success rates in this study are compared favourably with the 65% and 68% success rates reported previously with another PAT-MAD [6, 7]. No patient in the current study had problems with device retention confirming previous in vitro results which showed that this PAT-MAD possesses sufficient retention forces to resist initial jaw opening and probably full mouth opening [13]. The device was well tolerated and only mild AEs were reported (mainly dry mouth (37.5%), toothache (37.5%) and TMJ discomfort (16.7%)).
CONCLUSION
We conclude that this new PAT-MAD may be an effective short-term treatment for OSAHS, particularly in a resource-limited setting where custom-made devices are unavailable to many patients due to cost. Long term studies in a larger number of patients are warranted to assess the long-term efficacy, retention and side-effects of this device.
LIST OF ABBREVIATIONS
| AE | = Adverse event |
| AHI | = Apnoea-hypopnoea index |
| CPAP | = Continuous positive airway pressure |
| EOS | = End of study |
| ESS | = Epworth Sleepiness Scale |
| ODI | = Oxygen desaturation index |
| OSAHS | = Obstructive sleep apnoea-hypopnoea syndrome |
| PAT-MAD | = Prefabricated adjustable thermoplastic mandibular advancement device |
| PSG | = Polysomnography |
| TMJ | = Temporomandibular joint |
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors thank Newmed Publishing Services for support with the statistical analyses and writing of this letter. They also thank Dr Bernard Fleury of Saint Antoine Hospital, Paris, France, and Dr Roy Dookun of Cleveland House Dental Practice, Guernsey, for their comments on the final manuscript draft. BlueSom, France, provided the PAT-MADs free of charge.


