RESEARCH ARTICLE
Prevalence and Predictors of Ocular Complications in Obstructive Sleep Apnea Patients: A Cross-sectional Case-control Study
Nesreen E. Morsy1, 9, Badawi E Amani2, Ahmad A Magda1, Awadalla J Nabil3, Seithikurippu R. Pandi-Perumal4, Ahmed S. BaHammam5, David Warren Spence6, Per O. Lundmark7, Nevin FW Zaki8, 9, *
Article Information
Identifiers and Pagination:
Year: 2019Volume: 13
First Page: 19
Last Page: 30
Publisher ID: TORMJ-13-19
DOI: 10.2174/1874306401913010019
Article History:
Received Date: 16/02/2019Revision Received Date: 27/05/2019
Acceptance Date: 23/06/2019
Electronic publication date: 31/07/2019
Collection year: 2019

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose:
This study sought to identify the magnitude and the possible risk factors of ocular complications in patients with Obstructive Sleep Apnea (OSA).
Methods:
A hospital-based cross-sectional study with a nested case-control design was conducted. Qualifying study subjects were patients who had been diagnosed with moderate to severe OSA (AHI index of ≥ 5, n=80), and control subjects (n=20) who had an AHI index of ≤ 5 (“normal”). Study participants were recruited from Mansoura University Hospital’s Sleep Disorders Clinic in Mansoura, Egypt.Selected subjects were assessed for ocular complications at Mansoura Univerity Hospital Ophthalmic Center, (Mansoura), Egypt. An ophthalmic history was recorded, and opthalmic testing was carried out. The testing included unaided visual acuity measurement, refraction, best-corrected visual acuity measurement, slit lamp bio-microscopic evaluation of the anterior segment and anterior segment photography, dilated fundus examination, intraocular pressure measurement, fundus photo, and fluorescein angiography, and visual field assessment. Various tests of OSA symptoms were also monitored, including the AHI, lowest oxygen concentrations and desaturation index plus the overall severity index. .
Results:
It was found that OSA patients n=28 (35%), n=24 (30%), n=4 (5%) had senile cataract, normal tension glaucoma, and retinal ischemia, respectively, with an overall prevalence of 45%. Additionally, the OSA group had seven times greater risk (OR=7.36, 95%CI: 1.6-33.86) of vision-threatening disorders compared to the controls. OSA patients were observed to be at a greater risk of senile cataract 28 (35%), normal tension glaucoma 24 (30%), retinal ischemia 4 (5%) and conjunctival hyperemia and dry eye (OR=3.77, 95%CI: 1.02-13.95, OR=4.36, 95%CI: 1.26-17.08). Also, multivariate logistic regression analysis testing showed that the lowest oxygen saturation index was the only significant predictor negatively associated with vision-threatening disorders (OR=0.84, 95%CI: 0.75-0.93).
Conclusion:
The risk of vision-threatening and non-threatening ocular disorders is higher among OSA cases. The lowest oxygen saturation index was the only significant predictor of vision-threatening disorders. These findings support the recommendation that a full ophthalmic examination should be carried out on patients with confirmed OSA.
1. INTRODUCTION
Obstructive Sleep Apnea (OSA) is currently a global concern. It is defined as a prevalent sleep disorder affecting males more than females (34% versus 17% respectively) [1]; however, many cases remain undiagnosed. OSA is characterized by repeated episodes of apnea and hypopnea events followed by arousal and oxygen desaturation during sleep with sympathetic nervous system surges [2, 3]. These physiological disturbances often give rise to an increase in blood pressure, insulin resistance, changes in heart rate and arrythmias [4], endothelial dysfunction, systemic inflammatory markers [5, 6], release and enhanced platelets aggregations [7]. It is known that these are core mechanisms involved in the pathogenesis of cardiovascular disease (CVD), diabetes and other neurological disorders [8]. Although OSA is a well-known major risk factor for many systemic diseases and multi-organ dysfunction [9], a few studies have additionally reported that OSA is associated with eye disorders [10-12]. These studies suggested that the main mechanisms leading to the ocular complications of OSA are mainly intermittent hypoxia, sympathetic overstimulation, oxidative stress, and damaging effects of endothelin-1 (ET-1) [13].
The reported eye complications in patients with OSA include, Floppy Eyelid Syndrome (FES), keratoconus, retinal vascular tortuosity and congestion, non-arteritic anterior ischemic optic neuropathy, glaucoma, papilledema, the proportional decrease in retinal nerve fiber layer, higher incidence of visual field defects and reduced tear film break-up time, and lacrimal gland prolapse [10, 14]. The association between OSA and ocular diseases is being increasingly recognized as a critically important topic due to the irreversible consequences to normal eye functioning that may have been caused by OSA, and thus may have been preventable [15].
There is an urgent need for studies which clarify the relationship between OSA and eye disease. The need to predict ocular complications among OSA patients underscores the importance of requesting a full ophthalmic evaluation after confirmation of the diagnosis of the OSA. Furthermore, the acquisition of such information could be useful for determining which factors pose the greatest risk for producing ocular pathologies. Additionally, this information could be helpful for clinicians in developing treatment protocols for the primary and secondary prevention of such complications. We hypothesized that OSA patients might have specific ophthalmologic findings that represented possible risk factors or warning signs for further ocular disease development. This evidence in turn could aid somnologists in selecting which patients needed a referral to ophthalmologists for care and prevention of visual complications resulting from OSA. Additionally, no study has assessed the prevalence and predictors of ocular complications among Arabs, a factor which is relevant inasmuch asracial/ethnic differences in eye-related diabetic complications have been reported [16, 17]. Therefore, it is possible that reported ocular complications of OSA may not necessarily apply to Arabs. In view of the significant knowledge deficits in this area, the present study was designed to evaluate the prevalence and predictors of ocular complications in Egyptian (Arab) patients with OSA.
2. SUBJECTS AND METHODS
2.1. Study Sesign
A Hospital based cross-sectional and nested case-control study was conducted on 100 subjects who had undergone consultations for the possibility of OSA during the period from January 2014 to February 2015 in the Sleep Clinic of Mansoura University Hospitals. We adopted the STROBE strategy for reporting this observational study [18], and a flow chart summarizing the methodological steps used is provided in Fig. (1).
The study sample was made up of all subjects (n=100), who had attended the sleep disorders clinic during the study period, and who were clinically suspected to have OSA. The study patients were further classified into OSA patients and normal subjects depending on the results of the full night, in-laboratory polysomnographic testing; the sample was thus split according to the recorded apnea-hypopnea index (AHI<5, ≥5 respectively). The grades of OSA were defined as mild: AHI: 5-14.9, Moderate: AHI: 15-29.9, Severe: AHI: ≥30. These classifications were based on the diagnostic criteria of the third International Classification Of Sleep Disorders (ICSD-3) [19]. Normal subjects were designated as the control group. We excluded smokers, those with systemic disorders (such as systemic hypertension, diabetes mellitus, cerebrovascular disorders, etc.), and those on regularly prescribed medications (e.g., steroids, anti-inflammatory drugs, anticoagulants, anti-hypertensives, oral hypoglycemics, insulin, etc.). Details of the exclusion criteria are shown in Fig. (1).
This study was conducted in accordance with the Declaration of Helsinki, and written informed consent was obtained from all of the participants. The study protocol was reviewed and approved by the local Institutional Review Board (Approval No. # IRB: R/17.02.112) of the Faculty of Medicine, Mansoura University, Egypt.
2.2. Body Mass Index (BMI) Calculation
Weight and height were measured for all participants using a standardized weight-height scale, then BMI was calculated according to the WHO manual. Grades I, II and III obesity were considered when BMI ranged between 25-29.9, 30-34.9 and above 35 respectively [20].
2.3. History Taking and General Examination
History taking and examination were conducted to obtain the sociodemographic criteria of the studied groups such as: age, sex, occupation, smoking status, and duration of illness.
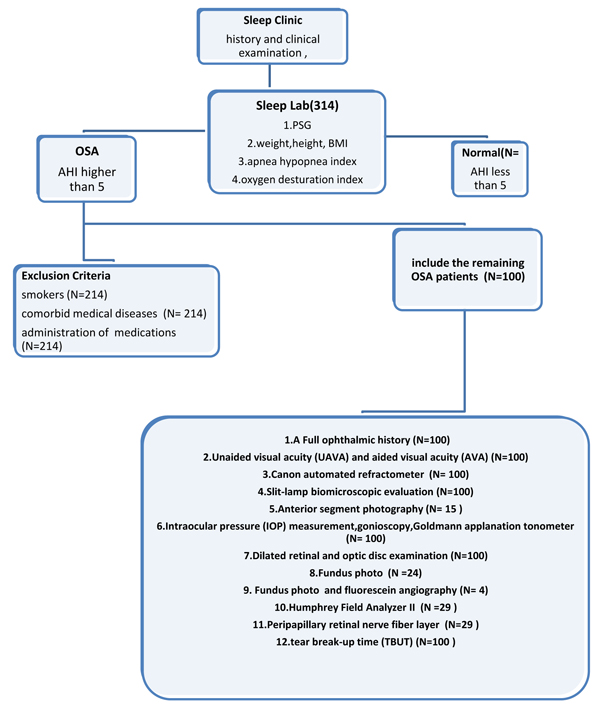 |
Fig. (1). A flow chart summarizing the methodological steps. |
2.4. Polysomnographic Evaluation
All subjects enrolled in this study were invited to undergo 1 full overnight in-laboratory polysomnography (SONMOscreenTM plus, SOMNOmedics GmbH, Germany). The American Academy of Sleep Medicine [21] standard montage was used, and the studies were scored according to the most recent scoring criteria using the recommended criteria for identifying sleep-related breathing [19]. Polysomnography was carried out using Somnoscreen plus RC Combi39 including the following channels: flow (cannula and thermistor), snoring (microphone), thoracic and abdominal effort (RIP), and oxygen saturation (SpO2). Measurement devices included the plethysmogram, pulse rate monitor, electrocardiogram (ECG), and electroencephalogram (EEG). The AHI was calculated as the number of apnea plus hypopnea episodes per hour of sleep. The oxygen desaturation index (ODI) was defined as the average number of oxygen desaturations of 4% or more per sleeping hour. The overall sleep apnea severity score adopted was calculated by combining AHI and ODI [22].
2.5. Ophthalmologic Examination
A comprehensive ophthalmologic evaluation was carried out for all participants by the second author in the next morning after polysomnography. Patients were sequentially referred to the Mansoura Ophthalmic Center after clinical evaluation at the sleep disorders unit of the Chest Department, Mansoura University.
- A full ophthalmologic history was taken to identify any irritating ocular symptoms (e.g., redness, tearing, foreign body sensation, eye rubbing, and discharge), spontaneous lid eversion and history of recent visual blurring. Furthermore, a comprehensive ophthalmologic examination, including a review of the patient's medical history, to exclude other differential diagnoses, was also performed.
- Unaided visual acuity (UAVA) and aided visual acuity (AVA) assessment were carried out using the Landolt C visual chart.
- Refraction was measured using the Canon automated refractometer (Canon R-30, USA).
- Slit-lamp biomicroscopic evaluation of the anterior segment focusing on eyelid anomalies, meibomian gland impairment, and ocular surface dysfunction or ectatic corneal diseases.
- Anterior segment photography: using a digital camera “Canon Power Shot A480 digital camera attached to the slit-lamp biomicroscopy.
- Intraocular pressure (IOP) measurement by the Goldmann applanationtonometry (GAT) apparatus.
- Dilated retinal and optic disc examination using stereoscopic biomicroscopy and none- contact Volk 90 lens.
- Fundus photo and fluorescein angiography (when indicated) using the Canon CF-1 Digital retinal camera (Canon Inc., USA).Visual field assessment in patients with suspected disc changes using Humphrey Field Analyzer II, central full threshold 30-2 visual field test. Glaucomatous visual field defects were defined as those who met two of the following criteria as confirmed by more than two reliable consecutive tests, in addition to compatibility with optic nerve appearance [23]: a cluster of three points with a probability of less than 5% on a pattern deviation map in at least one hemifield and including at least one point with a probability of less than 1% or a cluster of two points with a probability of less than 1% [23]; a Glaucoma Hemifield Test (GHT) result beyond 99% of the age-specific normal limit; and (3) a Pattern Standard Deviation (PSD) beyond 95% of the normal limit. Reliable visual field assessment was defined as a visual field test with a false-positive, a false-negative, and a fixation (a fixation loss of 20% or less and false negatives or positives of 15% or less based on the reliability indices were included in the analysis). The first perimetric result was excluded from the analysis to avoid lack of acclimatization effects. If the results were not reliable, the test was repeated within 2 weeks.
- Peripapillary Retinal Nerve Fiber Layer (RNFL) thickness using optical coherence tomography (OCT) was also measured in those patients to confirm glaucomatous changes.
- Normal Tension Glaucoma (NTG) was diagnosed if a subject showed glaucomatous visual field loss and a glaucomatous optic disc, using the method of Lim et al. [24]. Glaucomatous optic neuropathy was diagnosed using funduscopic examination, characteristic visual field defects, open normal anterior chamber angles on gonioscopy, and pretreatment IOP never exceeding 21 mmHg, as measured by the GAT. IOP was tested by the same ophthalmologist during the day, and the average of the 3 measurements was used (adapted from Kuerten et al.) [25].
A single follow-up visit was conducted after three months of initial diagnosis similar to the method of Hayamizu et al. [26]. Glaucoma was diagnosed according to the following characteristics:
- Average IOP with considerations of a single IOP measurement never exceeded 24 mm Hg, the median IOP values was less than 20 mm Hg, and nine IOP readings out of ten were 22 mm Hg or less [14].
- Lack of other primary or secondary pathologies of the optic disc changes; gonioscopy revealed normal open angle of the anterior chamber.
- Characteristic glaucomatous optic nerve head changes with neuroretinal rim loss.
- Compatible visual field defect with the optic disc damage and RNFL loss [27].
- Dry eye and tear film stability were diagnosed by Tear Break-up Time (TBUT): A fluorescein dye strip was applied into the inferior conjunctival sac. Following this the participants were asked to blink several times before holding the blinking. The corneal surface was then examined by the blue filter and a wide beam of the slit lamp microscopy. The time between the last blinking and the first corneal dry spot appearance was estimated. A TBUT score of less than 10 seconds was considered to be an abnormal value with an indication of tear film instability [28].
- RNFL was measured using the fast RNFL mode of OCT, and the average RNFL thickness results were used for the analysis. The occurrence of OCT images with a signal strength of 6 or above with the optic disc in the center of the scan circle were considered reliable criteria and were included in the analysis. Floppy Eyelid Syndrome (FES) was diagnosed subjectively by gross examination of the lid with upward pulling on it or the lashes. Spontaneous easily everted eyelid, with conjunctival exposure, papillae, hyperemia, and/or signs of inflammation were considered supportive of the FES diagnosis [29]. Diagnosis of cataract was done using slit-lamp examination only since the grading of cataract was not indicated, and no further measurements were done.
2.6. Statistical Analysis
All statistical analyses were carried out using the IBM SPSS Statistics v.23.0 package for Windows (IBM Corp., Armonk, NY, USA) [3]. Descriptive statistics were presented as number and percentage for categorical data and as means and Standard Deviations (SD) for continuous data. Chi-Square tests were used for the association between categorical variables, and an independent t-test was used for continuous data. Significant factors predicting ocular disorders among OSA patients on a univariate analysis model were entered into a multivariate logistic regression analysis to establish the independent possible risk factors for ocular disorders in OSA patients. Odds Ratios (ORs) and 95% Confidence Intervals (CIs) were calculated. P < 0.05 was considered statistically significant.
3. RESULTS
Full ophthalmic examinations were carried out for 80 confirmed OSA patients (all were moderate and severe grades with no mild case), and 20 healthy control subjects. Demographic profiles of the recruited sample are summarized in Table 1. Importantly, and as shown in Table 1, no statistically significant difference was observed between both groups of patients regarding the basal oxygen level. On the other hand, AHI, lowest oxygen desaturation index, desaturation index, total severity index and arousal index were significantly higher in OSA cases compared with the control subjects (P =0.001 for all).
The multivariable logistic regression (Table 2) model for prediction of ocular complication among the study groups revealed that being an OSA patient was the only significant predictor for developing ocular complications (aOR=3.30, 95%CI: 1.13 -9.58); thus, we conducted further analysis of the study variables that were presented in the results.
As summarized in Tables 3-7, multivariate logistic regression analysis revealed that OSA patients were at a greater risk of conjunctival hyperemia and dry eye (OR=3.77, 95%CI: 1.02-13.95 and OR=4.36, 95%CI: 1.26-17.08 respectively) when compared to controls. Also, 28 (35%), 24 (30%) and 4 (5%) OSA patients had senile cataract, glaucoma, and retinal ischemia, respectively, with an overall prevalence of 45% for vision-threatening disorders and 7 times greater risk (OR=7.36, 95%CI: 1.6-33.86) compared with controls. The significant clinical and polysomnographic variables of each disorder are represented in Tables 4 and 5. It was found that low oxygen saturation was a significant predictor for the occurrence of all studied diseases, except conjunctival hyperemia. OSA variables including the AHI, basal oxygen level, oxygen desaturation index, total severity index, arousal index were significantly in the abnormal range most of ocular diseases studied.
| Demographic | Controls (N=20) N(%) |
Patients (N=80) N(%) |
p-value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 11 (55) | 44(55) | 1 | |
| Female | 9 (45) | 36(45) | ||
| Age group | ||||
| <30 | 3(15) | 8(10) | 0.66 | |
| 30-50 | 10(50) | 36(45) | ||
| >50 | 7(35) | 36(45) | ||
| Occupation | ||||
| Blue collar worker | 2(10) | 17(21) | 0.53 | |
| Farmer | 4(20) | 11(13.8) | ||
| Housewife | 4(20) | 24(30) | ||
| White collar workers | 9(45) | 24(30) | ||
| Driver | 1(5) | 4(5) | ||
| Obesity grade | ||||
| Obesity grade I | 1(5) | 1(1.3) | 0.55 | |
| Obesity grade II | 4(20) | 15(18.8) | ||
| Obesity grade III | 15(75) | 64(80) | ||
| Duration of illness | ||||
| <2 years | 11(55) | 47(58.8) | 0.34 | |
| from 2-4 years | 4(20) | 23(28.7) | ||
| >4 years | 5(25.0%) | 10(12.5) | ||
| Variables | B | S.E. | AOR(95%CI) |
|---|---|---|---|
| OSA patients vs. Controls | 1.19 | 0.54 | 3.30(1.13-9.58)* |
| Age (years) | 0.03 | 0.02 | 1.03(0.98-1.08) |
| Sex (Female vs. Male) | -0.12 | 0.58 | 0.89(0.28-2.75) |
| Smoking (smoker vs. Non-smokers | 0.443 | 0.71 | 1.56(0.38-6.29) |
| BMI | 0.04 | 0.01 | 1.12(0.89- 1.45) |
| Polysomnography Criteria | Controls(N=20) | Patients(N=80) | P-Value |
|---|---|---|---|
| Apnea–Hypopnea Index | 2.65±1.23 | 42.45±26.96 | 0.001* |
| Basal oxygen level | 95.30±2.00 | 94.90±2.93 | 0.56 |
| Lowest oxygen saturation | 92.05±1.88 | 82.00±10.37 | 0.001* |
| Desaturation index | 1.00± 0.97 | 43.70±31.04 | 0.001* |
| Total severity index | 3.65± 1.53 | 86.15±57.45 | 0.001* |
| Arousal index | 43.70±31.04 | 36.45±27.02 | 0.001* |
| Disorders | Controls (N=20) N(%) |
OSA Cases (N=80) N(%) |
OR (95%CI) N(%) |
|---|---|---|---|
| Non-vision threatening ocular disorder | 10(50) | 56(70) | 2.33(0.86-6.33) |
| Floppy eyelid | 2(10) | 16 (20) | 2.25 (0.47-10.71) |
| Conjunctival hyperemia | 3(15) | 32(40) | 3.77(1.02-13.95)* |
| Conjunctival Chalsia | 2(10) | 16 (20) | 2.25(0.47-10.71) |
| Dry Eye | 3(15) | 36 (45) | 4.63(1.26-17.08)* |
| Vision threatening ocular disorder | 2(10) | 36(45) | 7.36(1.60-33.86)* |
| Senile cataract | 2(10) | 28(35) | 4.84(1.05-22.41)* |
| Normal-tension Glaucoma | 0 (0) | 24 (30) | - |
| Non-Arteritic Anterior Ischemic Optic Neuropathy | 0(0) | 0(0) | - |
| Papilledema | 0(0) | 0(0) | - |
| Retinal ischemia | 0(0) | 4(5) | - |
| Factors | Conjunctival hyperemia | Dry eye | ||||
|---|---|---|---|---|---|---|
|
`Absent Mean ±SD |
Present Mean ±SD |
P-Value |
`Absent Mean ±SD |
Present Mean ±SD |
P-Value | |
| Age (year) | 46.5± 10.6 | 45.5± 8.11 | 0.34 | 43.09 ±10.18 | 49.78± 7.56 | 0.008* |
| Sex | 0.36 | 28(63.3%) | 16(36.4%) | 0.11 | ||
| Male | 24(54.5%) | 20(45.5%) | 16(44.4%) | 20(56.6%) | ||
| Female | 24(66.6%) | 12(33.3% | ||||
| Body Mass Index | 43.31± 4.81 | 42.38± 3.74 | 0.46 | 43.2±4.47 | 42.61± 4.38 | 0.401 |
| Duration of illness (years) | 2.71± 1.4 | 1.40 ±1.72 | 0.34 | 2.61 ±1.5 | 2.69± 1.58 | 0.81 |
| Apnea–Hypopnea Index | 37.42± 21.18 | 50 ±32.77 | 0.12 | 36.64± 22.6 | 49.56± 30.31 | 0.03* |
| Basal oxygen level | 95.42 1.87 | 94.13 ±3.95 | 0.3 | 95.73 1.98 | 93.89± 3.56 | 0.02* |
| Lowest oxygen sat. Index | 85.17 ±6.89 | 77.25±12.78 | 0.03* | 87.55± 6.74 | 75.22±10.05 | 0.001* |
| Desaturation index | 39.33 ±25.44 | 50.25±37.43 | 0.53 | 34.91±28 | 54.44 31.55 | 0.01* |
| Total severity index | 33.5 ±17.29 | 40.8 ±37.06 | 0.81 | 27.64± 17.55 | 47.22±32.44 | 0.01* |
| Arousal index | 76.75 ±45.95 | 100.2 69.7 | 0.27 | 71.55±49.68 | 104±61.81 | 0.03* |
A multivariate logistic regression analysis showed that a low oxygen saturation index was the only significant possible predictor that was negatively associated with both senile cataract and NTG (AOR=0.74, 95% CI: 0.63-0.85 and AOR=0.82, 95%CI 0.71-0.94 respectively). On the other hand, AHI was found as a significant possible risk factor that was positively associated with NTG (AOR= 1.10, 95%CI 1.04-1.17) as shown in Table 6.
Characteristics of OSA patients, either with or without NTG, and controls are shown in Table 8. Among patients who had NTG the mean IOP was 16.14±1.32 mmHg, while the mean IOP in controls was 15.45±(1.41) (P-value =0.325). The mean Cup-Disc (C/D) ratio was 0.671± 0.21 and 0.243±0.213 in glaucoma and controlled groups respectively. In the visual fields of glaucomatous patients, the mean deviation value was -6.12 ± 2.01decibels, while the mean pattern standard deviation value was 6.51±1.44 decibels. The visual fields in the control group were within average.
| Factors | Senile cataract | Normal tension glaucoma | ||||
|---|---|---|---|---|---|---|
|
`Absent Mean ±SD |
Present Mean ±SD |
P-Value |
`Absent Mean ±SD |
Present Mean ±SD |
P-Value | |
| Age | 44.23±10.86 | 49.57±6.51 | 0.005* | 45.07±10.64 | 48.50±6.32 | 0.12 |
| Sex | 44.23±10.86 | 49.57±6.51 | 0.005* | 45.07±10.64 | 48.50±6.32 | 0.12 |
| Male | 28(53.8%) | 16(57.1%) | 0.81 | 32(57.1%) | 12(50%) | 0.62 |
| Female | 24(46.2%) | 12(42.9%) | 24(42.9%) | 12(50%) | ||
| Body Mass Index | 42.80± 4.58 | 43.17±4.24 | 0.57 | 42.96±4.62 | 42.88±4.01 | 0.98 |
| Duration of illness (years) | 2.57±1.62 | 2.78±1.34 | 0.294 | 2.63±1.45 | 2.71±1.73 | 0.94 |
| Apnea–Hypopnea Index | 37.46±25.06 | 51.71±28.33 | 0.03* | 30.93±18.87 | 69.33±23.82 | 0.001* |
| Basal oxygen level | 95.53±2.70 | 93.71±3.01 | 0.001* | 95.79±6.63 | 92.83±3.51 | 0.001* |
| Lowest oxygen sat. Index | 85.76±8.29 | 75.0±10.32 | 0.000* | 86.79±6.63 | 70.83±8.85 | 0.001* |
| Desaturation index | 39.15±30.66 | 52.14± 30.48 | 0.08 | 30.64±20.12 | 74.17±30.95 | 0.001* |
| Total severity index | 29.23±20.92 | 49.85±31.96 | 0.003* | 29.50±20.33 | 52.67±33.57 | 0.001* |
| Arousal index | 76.61±55.23 | 103.85±58.24 | 0.04* | 61.57±38.39 | 143.50±53.91 | 0.001* |
| Polysomnographic parameter | Senile cataract | Normal tension glaucoma | ||||
|---|---|---|---|---|---|---|
| B | S.E. | AOR(95%CI) | B | S.E. | AOR(95%CI) | |
| Apnea–Hypopnea Index | 0.06 | 0.04 | 1.06(0.99-1.14) | 0.10 | 0.03 | 1.10(1.04-1.17)* |
| Basal oxygen level | -0.30 | 0.17 | 0.74(0.52-1.04) | -0.28 | 0.29 | 0.99(0.78-1.18) |
| Lowest oxygen sat. Index | -0.31 | 0.07 | 0.74(0.63-0.85)* | -0.20 | 0.07 | 0.82(0.71-0.94)* |
| Desaturation index | -0.20 | 0.09 | 0.82(0.73-1.04) | 0.01 | 0.01 | 1.02(0.99-1.03) |
| Arousal index | 0.11 | 0.04 | 1.12(1.040-1.20) | -0.06 | 0.03 | 0.94(0.90-1.00) |
| Constant | 0.06 | 18.810 | - | 12.87 | 5.72 | - |
| R2 | 59.80% | 75.7% | ||||
| Parameter | OSA subjects with NTG (n=24) | OSA subjects without NTG (n=56) | Controls (n= 20) |
P values |
|---|---|---|---|---|
| Mean IOP (mm Hg) |
16.14 ± 1.32 | 15.66 ± 1.02 | 15.45 ± 1.41 | .082 |
| C/D ratio | 0.671± .21 | 0.231 ± .44 | 0.243±.213 | 0.001* |
| MD (Decibels) |
-6.12 ± 2.01 | -1.20 ±.82 | -1.12±.66 | 0.003* |
| PSD (Decibels) |
6.51±1.44 | 1.42±.77 | 1.35±.58 | 0.001* |
| Parapapillary RNFL thickness (total) | 69.34±11.33 | 112±9.92 | 115±12.52 | 0.001* |
| SE | -1.11±1.7 | -1.01±.78 | .98±.35 | .103 |
Floppy Eyelid Syndrome (FES) was detected in 16 (20.0%) of the total patients and 6.66% in severe cases. Conjunctival hyperemia and congestion were in 32(40.0%) of the total number, (30%) in the mild-moderate cases and 43.33% in severe cases
Decreased TBUT was diagnosed in 36(45.0%) of all patients, in (30%) of mild-moderate and (33.33%) in severe cases. Peripheral retinal non-perfusion (ischemic changes) was present in 4 patients suffering from old central retinal vein occlusion. It represented 5.0% of all studied patients. Three eyes showed peripheral ischemia less than 5 Optic Disc (OD) diameter area, and only one eye shows extensive peripheral ischemia (more than 5 OD diameter Table 8.
4. DISCUSSION
Several ocular complications have been found in association with OSA, including vision-threatening and non-threatening disorders. We conducted an ocular examination for OSA subjects and found harmful effects of the sleep disorder on patient’s vision. Many eye disorders such as glaucoma, optic neuropathy, papilledema, anterior ischemic optic neuropathy and floppy eyelid syndrome, formerly thought to be secondary to increased intracranial tension [30, 31]. The finding that symptoms of OSA are associated with ocular diseases and may possibly be predictors of these pathologies is clinically relevant and important due to the irreversible consequences of OSA that are possibly preventable [15].
4.1. Retinal Vein Occlusion
In this study, patients with moderate and severe OSA were found to exhibit a number of ocular manifestations. OSA patients were observed to be at a greater risk of conjunctival hyperemia and dry eye (Suppl Fig. 1). Additionally, we found that, compared to controls, OSA patients had more senile cataract (Suppl Fig. 2 & 3), glaucoma, and peripheral retinal ischemia due to retinal vein occlusion (RVO), with an overall prevalence of 45% and 7 times greater risk of vision-threatening disorders. . After diabetic retinopathy, retinal vein occlusion is deemed to be the most prevalent of retinal vascular disorders that leads to visual loss. As described above, OSA was found more frequently among patients with RVO when compared to those without RVO (77% vs. 37%) [11].
It is known that the pathogenesis of RVO is multifactorial and complicated. The core symptoms which are common to the largest number of RVO patients according to a study by Alashwal et al. [32] have been summarized as “Virchow's triad”: these three essential symptoms are hemodynamic changes, hypercoagulability, and endothelial dysfunction [33]. The persistence of these changes in many RVO patients have thus been hypothesized to play a role in the pathophysiology of RVO in OSA patients, inasmuch as they co-occur in both conditions. As OSA manifests, refined nocturnal hypercapnia gives rise to vasodilatation, reduction in venous return and therefore a decrease in blood flow; while the micro-arousal phase causes sharp alterations in BP. Also, the inflammation and oxidative stress processes increase the hypercoagulability status [13].
4.2. Vascular and Inflammatory Changes
OSA is linked to the accumulation of various inflammatory cells in the vascular endothelium and undue platelet activation and enhanced platelets aggregations [34]. It has been suggested that this adverse state might play a crucial role in the development of atheromatous plaque with its attendant cardiovascular consequences [13]. If supported by further findings of the occurrence of this pathological phenomenon in OSA patients, it could elucidate the mechanism accounting for the frequently observed correlation between OSA and venous occlusion.
The etiology of these different ocular complications is uncertain, however the present study has pointed to a possible contributing factor. The multivariate logistic regression analysis showed that the lowest oxygen saturation index was the only significant possible risk factor that was negatively associated with vision-threatening disorders (Suppl Fig. 2). Therefore we suggest that chronic intermittent hypoxia may be a significant risk factor for the development of vision-threatening disorders. This suggestion is supported by the previously reported finding of Pérez-Rico et al. [35] who concluded that the pathogenesis of ocular complications in OSA is most probably due to multifactorial causes. Both mechanical and vascular factors have been thought to be implicated in optic nerve pathology. The vascular factors mainly result from frequent or long episodes of hypoxia, which involve direct damage to the optic nerve, inflammation, oxidative stress, increased blood vessel resistance, lower cerebral perfusion, increased intracranial pressure, and autonomic dysfunction [35]. Recurrent extended upper respiratory obstruction and subsequent arousal produce a rise in sympathetic tone, consequently leading to activation of the Renin-Angiotensin System (RAS). In addition, the impact of associated hypoxia leads to an increase in blood pressure and resistance to blood vessels, causing damage to the endothelial lining of the blood vessels. Hence, disturbance of autonomic functions and a disparity between vasoconstriction and vasodilatation might occur. Moreover, reperfusion may have harmful effects during the arousal periods. These effects may take the form of increased inflammation and oxidative stress with increased inflammatory markers and reactive oxygen kinds [35].
4.3. Floppy Eye Lid Syndrome
The ocular complications in this study were within the prevalence range that has been reported in previous studies. The Floppy Eyelid Syndrome (FES) was diagnosed in 16 out of 80 studied cases (20.0%) that are in the precise range (2.2-32%) that have been found in other studies or case series of OSA patients. Mohamed and Massoud found that FES occurred in 3 (10%) OSA patients [36]. Other relevant studies include Roberts et al. 1/46, 2.2% [37]; Karger et al. (1/44, 2.3% [38]; McNab 3/20, 15% [39]; and in 1999 Mojon et al. [40] who found 14/44 (32%) patients with OSA had FES.
Floppy eyelid syndrome is a common lid disorder that is found more frequently in obese patients due to the weak tarsus. A normal person usually wakes up with mechanical pressure on his open eye from bed sheets or pillows, while in patients with sleep apnea; due to a reduced cortical arousability the eyelid stays open even with mechanical stress during sleep. With the passage of time, the lid becomes more lax and easily everted with little lateral traction.
4.4. Glaucoma
In this study, glaucoma was diagnosed in 24 out of 80 (30.0%) patients. Despite the finding in a number of studies of significant correlations between glaucoma and OSA, the hypothesis that OSA may contribute to glaucoma’s pathogenesis has been extensively studied.The association between glaucoma and OSA found in the present study was consistent with the results of those of Waller et al. who found that, among 100 patients with OSA, the prevalence of glaucoma was 27% [41]. Other studies have also shown that glaucoma is highly prevalent in patients with OSA. One Chinese study revealed that patients with OSA were four times more likely to have glaucomatous optic disc changes and visual field defects than age-matched controls [42]. Another study by Mojon et al. showed that the prevalence of glaucoma among 69 patients with obstructive sleep apnea was 7.2% [43]. Importantly, there is a strong relationship between glaucoma and OSA. In view of the strong association between OSA and glaucoma, a cautious approach would be to have all OSA patients screened for glaucoma. Further, OSA should be considered as a possible pathogenic contributor in all patients with primary open angle or normal tension glaucoma. These findings are very much similar to tin et al. [44] studies conducted worldwide indicating that despite the diversity of study samples due to geographic location, culture, and ethnicity, OSA has been consistently linked with adverse effects on the vision of patients affected by it.
4.5. Optic Neuropathy
The pathogenesis of optic neuropathy in OSA is most likely due to both vascular and mechanical elements that mostly result from repeated and prolonged periods of hypoxia [45]. It is a reasonable inference that vascular effects have a more influential impact on the pathogenesis of NTG more than mechanical factors.
OSA produces precise vascular alterations and disturbance in the retina and Optic Nerve Head (ONH). Steady and regular blood flow to both retina and ONH is necessary for their high metabolic demands. Thus inadequate blood supply caused by OSA interferes with the nourishment of ONH and RNFL and thence leads to their apoptosis [46, 47]. Additionally, the resulting hypoxia leads to oxidative stress and the imbalance between vasodilator and vasoconstrictor chemical mediators. Higher levels of endothelin-1 (a potent vasoconstrictor) and reduced availability of nitric oxide (a vasodilator) produce increased vascular impairment and a corresponding loss of cells [48, 49].
It was speculated that the RNFL cells are extremely sensitive to normal blood perfusion and a rebate in O2 saturation that could explicate the interrelation between the OAS and glaucoma [50].
One of the recent theories of NTG pathogenesis is that unequal pressure on both sides of lamina cribrosa produces damage to the Retinal Nerve Fiber Layer (RNFL), with evident lower IOP in OSA patients [51]. The thinning of the RNFL occurs more frequently in OSA patients than in normals, regardless of IOP or body mass index [52]. Moreover, Lin et al. concluded that there was a strong association between the severity of OSA and the incidence of NTG [53]. This association might explain the findings of the current study that NTG was highly prevalent among patients with severe OSA.
The current study did not detect Non-arteritic Anterior Ischemic Optic Neuropathy (NAION). Mohamed and Massoud found the prevalence of NAION to be 13.33% among OSA patients [36]. On the other hand, the prevalence of OSA among those with NAION has been reported to range from 71-89% [54]. Both Arda and her colleagues [15] and Bilgin et al. [55] found a significant relationship between NAION and OSA in their studies. They suggested that OSA is a contributing factor along with other risk factors, rather than being an independent one.
The significance of the possible relationship between OSA and NAION lies in the fact that there is no currently proven treatment method for NAION, so clinical management has focused on treating OSA to minimize the risk of the involvement of the second eye. In view of the condition’s seriousness we urgently recommend that confirmation by ophthalmologic evaluation that NAION is present, that affectred patients be screened for OSA (Suppl Fig. 4A and 4B).
4.6. Papilledema
In this study, papilledema was not found to be present in any patients. This may be attributed to the relatively small sample size. The association between papilledema and OSA was elucidated previously by a nocturnal increase in intracranial pressure. Probable mechanisms include the forcible inspiration against an obstructed airway as well as the effect of hypoxia on cerebral venous dilation. All lead to an increase in venous pressure. Precise history taking is critical in papilledema patients with normal neuroimaging to find out if OSA is a causative agent or not. It was reported that the proper treatment of OSA in those patients improved or halted the papilledema progression [10]. Sugita et al. monitored intracranial pressure for 24-h in patients with OSA and found that intracranial pressure was elevated during sleep in association with apnea, and that apneas of longer duration lead to a higher increase in ICP [56]. Episodes of apnea might precede and accompany episodes of intracranial pressure rise. Sugita et al. supposed that the dull morning headaches, which are frequent in patients with OSA may be due to an increase of cerebrospinal fluid pressure during sleep [56]. None of those patients experienced increased intracranial during the daytime. However, Waller et al. revealed that low blood oxygen and increased intracranial pressure might exist even in the daytime in some patients with severe OSA [41]. Purvin et al. reported papilledema in four patients with OSA [57]. They hypothesized that a decrease in blood oxygen level and a rise in CO2 would lead to cerebral vasodilation resulting in a transitory intracranial pressure elevation.
In the present study, 18 (60%) patients had dry eye manifestations in the form of chronic conjunctival hyperemia, lid-parallel conjunctival folds, and conjunctivochalasis (redundant conjunctiva). However, no previous reported studies have documented these ocular complications. Future research should explore the existence of this complication in other cultures and ethnicities.
5. STRENGTHS AND LIMITATIONS
We were able to assess the frequency of vision-threatening and non-threatening disorders for the first time among Arab patients with OSA through standardized methods and clinically by a well-trained ophthalmologist. Additionally, the possible risk factors of ocular disorders among candidate patients were explored. Further, a logistic regression analysis was carried out to determine which independent factors might predict the occurrence of vision threatening disorders. The goal of the study was to provide heuristics that could alert sleep medicine clinicians to the possible presence of eye problems, which in turn could be the basis for referrals for ophthalmic examinations of affected patients. The study however had certain limitations which may have affected the generalizability of the results to all OSA patients. In as much as this was a a cross-sectional study, the temporality of the associations between OSA and the related ocular problems make it difficult to confirm the causality or directionality of the suggested effects.
CONCLUSION
The risk of vision non-threatening ocular disorders (including conjunctival hyperemia and dry eye) and vision-threatening disorders (including cataract, glaucoma, and retinal ischemia) are greater among patients with OSA. Risk factors for non vision threatening disorders were detected (age AHI, basal oxygen saturation, lowest oxygen saturation idex, desaturation index, arousal index overall severity index), and the independent risk factors of vision threatening disorders (senile cataract and normal tension glaucoma) were AHI and lowest oxygen saturation index. Adequate treatments of OSA together with adequate eye follow-up are targets of future research and implementations to protect vision.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol was reviewed and approved by the local Institutional Review Board(Approval No. # IRB: R/17.02.112) of the Faculty of Medicine, Mansoura University, Mansoura, Egypt.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Written informed consent was obtained from all of the participants when they were enrolled.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The manuscript has been read and approved by all the authors, the requirements for authorship as stated in this document have been met and each author believes that the manuscript represents honest work.
ACKNOWLEDGEMENTS
We are extremely grateful to the study participants who took the time from their busy schedules to participate in the study. Without their participation; this study would not have been possible. The authors wish to acknowledge the efforts of our technical staff (Ms SamiaArafa, Ms Zeinab Mohammed, Mr Mohammed Ali) for their help and support throughout the study time.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers Website along with the published article.








