All published articles of this journal are available on ScienceDirect.
High Flow Nasal Cannula as Support in Immunocompromised Patients with Acute Respiratory Failure: A Retrospective Study
Abstract
Introduction:
High Flow Nasal Cannula (HFNC) is a novel technique for respiratory support that improves oxygenation. In some patients, it may reduce the work of breathing. In immunocompromised patients with Acute Respiratory Failure (ARF), Non-Invasive Ventilation (NIV) is the main support recommended strategy, since invasive mechanical ventilation could increase mortality rates. NIV used for more than 48 hours may be associated with increased in-hospital mortality and hospital length of stay. Therefore HFNC seems like a respiratory support alternative.
Objective:
To describe clinical outcomes of immunocompromised patients with ARF HFNC-supported.
Methods:
Retrospective study in patients admitted with ARF and HFNC-supported. 25 adult patients were included, 21 pharmacologically and 4 non- pharmacologically immunosuppressed. Median age of the patients was 64 [60-76] years, APACHE II 15 [11-19], and PaO2:FiO2 218 [165-248]. Demographic information, origin of immunosuppression, Respiratory Rate (RR), Heart Rate (HR), Mean Arterial Pressure (MAP), oxygen saturation (SpO2) and PaO2:FiO2 ratio were extracted from clinical records of our HFNC local protocol. Data acquisition was performed before and after the first 24 hours of connection. In addition, the need for greater ventilatory support after HFNC, orotracheal intubation, in-hospital mortality and 90 days out-patients’ mortality was recorded.
Results:
Mean RR before the connection was 25±22 breaths/min and 22±4 breaths/min after the first 24 hours of HFNC use (95% CI; p=0.02). HR mean before connection to HFNC was 96±22 beats/min, and after, it was 86±15 beats/min (95%CI; p=0.008). Previous mean MAP was 86±15 mmHg, and after HFNC, it was 80±12 mmHg (95%CI; p=0.09); mean SpO2 after was 93±5% and before it was 95±4% (95% CI; p=0.13); and previous PaO2:FiO2 mean was 219±66, and after it was 324±110 (95%CI; p=0.52). In-hospital mortality was 28% and 90 days out-patients’ mortality was 32%.
Conclusion:
HFNC in immunosuppressed ARF subjects significantly decreases HR and RR, being apparently an effective alternative to decrease work of breathing. In-hospital mortality in ARF immunosuppressed patients was high even though respiratory support was used. Better studies are needed to define the role of HFNC-support in ARF.
1. INTRODUCTION
Acute Respiratory Failure (ARF) remains the most common clinical condition in immunosuppressed patients, being the first cause of admission to Intensive Care Units (ICU) [1]. The more prevalent etiology is bacterial infections and pneumonia. Azoulay, in the prospective multinational study Efraim, reported mortality in ICU close to 50% which could reach up to 90% if invasive mechanical ventilation (IMV) is required, mainly due to nosocomial infections, frailty and immuno- suppression [2]. In recent years, evidence has described that in this population, there is usually a delay in admission to the ICU [2].
Supportive strategies, such as non-invasive ventilation (NIV), have been found to be effective in reducing intubation rates and thus reducing mortality in immunosuppressed patients with ARF [3-5]. However, the use of NIV for more than 48 hours is associated with increased respiratory failure and reduced survival since lung injury is related to the use of high ventilator pressures and high minute volumes that are common indicators of an increased work of breathing (WOB) [6, 7]. The literature is not conclusive about NIV use for hypoxemic failure, showing rates up to 50% of failure in non-hypercapnic patients [8-11]. The Consensus “Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure” (Rochwerg, 2017) shows a low quality evidence for supporting the use of NIV in immunocompromised patients.
HFNC generates a low positive end-expiratory pressure (less than 4 cmH2O) and reduces physiological dead space, lowering high airway carbon dioxide (CO2) concentration known as the washout effect [8, 9, 12, 13]. Another observed effect is a significant increase in end-expiratory lung volume compared with conventional oxygen therapy. This suggests an increase in functional residual capacity [14, 15] and, therefore, WOB reduction to be associated with a lower Respiratory Rate (RR) and Heart Rate (HR) [9, 16]. Also, HFNC delivers heating and humidified inspire gas that improves comfort and therapy adherence, being better tolerated than the NIV interface [16].
Frat (2015) compared HFNC, conventional oxygen therapy (COT) and NIV; The intubation rate was 38% in the HFNC group, 47% in the COT group and 50% in the NIV group. The number of ventilator-free days at day 28 was significantly greater in the HFNC group. Frat (2015) also described an HR for death at 90 days of 2.01 with COT, and an HR of 2.5 with NIV, versus HFNC [8]. In addition, Coudroy (2016) reported a significant decrease in mortality and intubation rate at 28 days in HFNC-supported patients versus those who were connected to NIV (55 vs. 35%) [17].
They suggest a possible negative effect of NIV use in immunocompromised patients [17, 18]; nevertheless, new evidence describes that independently of the chosen respiratory support, mortality in immunocompromised patients is still an independent variable. Despite all, NIV continues to be the mainly recommended treatment for immunocompromised patients with ARF. Frat (2019) suggests that further studies should evaluate which is the better strategy between HFNC versus NIV for immunocompromised patients, mostly because this population has not yet been completely described [18]. There is still a lack of evidence supporting HFNC use in immunocompromised patients and more studies are required to validate its use.
This study aims to describe clinical behavior in HFNC-supported pharmacological and non-pharmacological immuno- compromised patients with ARF.
2. METHODS
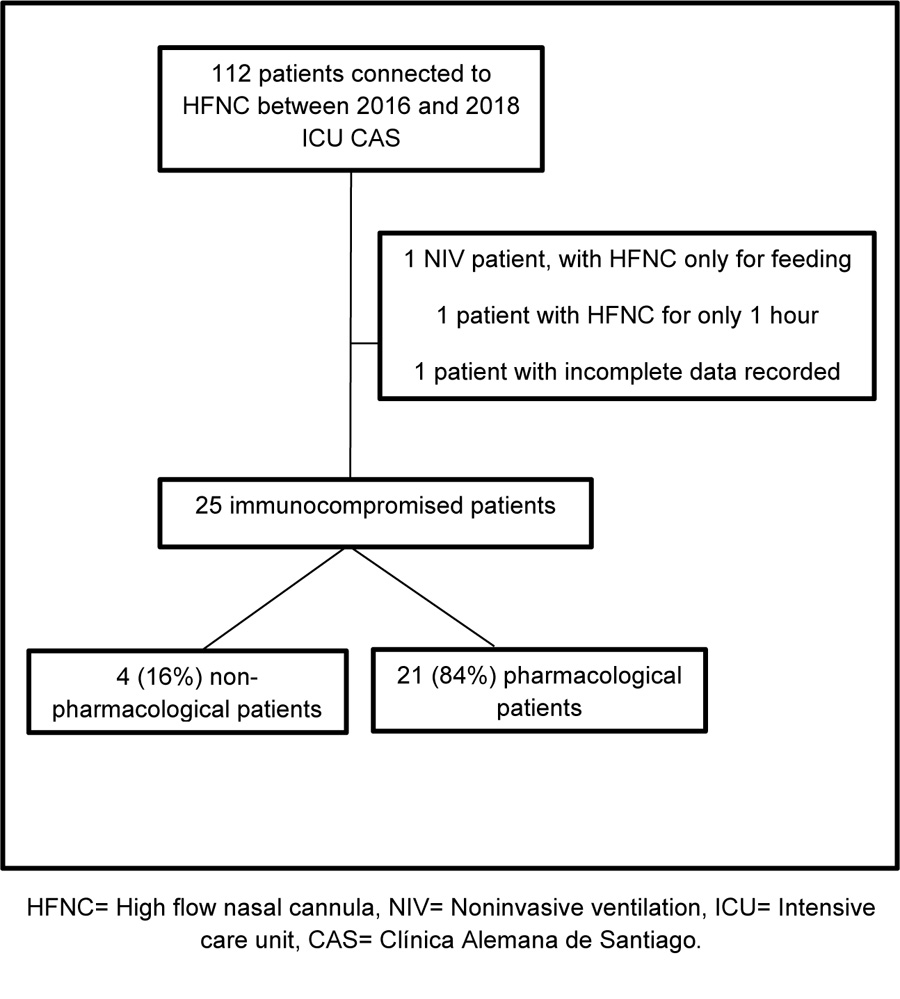
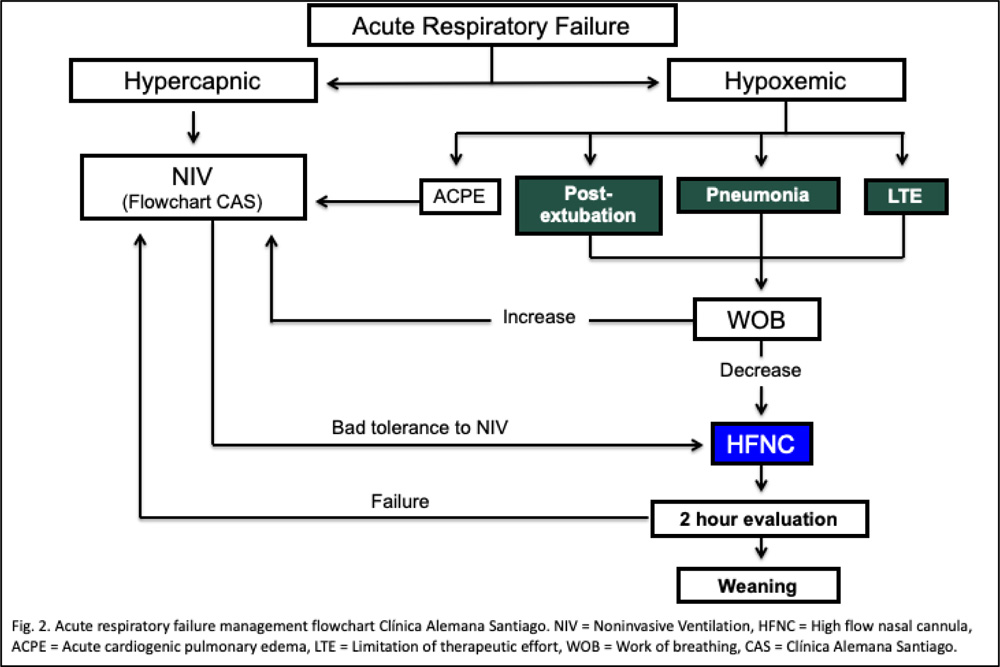
An observational retrospective study on pharmacological and non-pharmacological immunocompromised subjects with ARF diagnosis and HFNC-supported was conducted between January 2016 and July 2018. Inclusion criteria was decided according to our HFNC internal protocol. The exclusion criteria were 1) Incomplete data registered, and 2) Intermittent HFNC use (less than 3 hours daily use) (Fig. 1). Our HFNC internal protocol states that patients with ARF should be connected to HFNC, if with the following diagnosis: hypoxemic ARF, weaning from IMV and NIV, patients with hypercapnic ARF who do not tolerate NIV, to provide comfort to patients with limited therapeutic effort (Fig. 2).
| Demographics | Title |
|---|---|
| Age, years, Median (IQR) | 64 (60-76) |
| Sex, Male, n (%) | 17 (68%) |
| Immunosuppression cause, Oncological, n (%) | 21 (84%) |
| Acute respiratory failure at ICU admission, n (%) | 19 (76%) |
| APACHE II score, Median (IQR) | 15 (11-19) |
| PaO2:FiO2 ratio, Median (IQR) | 219 (166-249) |
| Hospital survival, n (%) | 18 (72%) |
| HFNC connection cause | |
| Hypoxemic failure, n (%) | 16 (64%) |
| Hypercapnic failure, n (%) | 1 (4%) |
| IMV weaning, n (%) | 1 (4%) |
| NIV weaning, n (%) | 5 (20%) |
| NIV intolerance, n (%) | 2 (8%) |
Initial HFNC was set at a flow of 60 Lt/min and was lowered according to patient tolerance. The temperature was set at 37ºC and was lowered to 34ºC or 32ºC according to patient tolerance. FiO2 was set to provide a SpO2 ≥ 90%. For HFNC weaning, the flow was lowered to ≤ 35 Lt/min and FiO2 ≤ 35% with respect to patients’ tolerance and acceptable clinical criteria. The HFNC device used was an Airvo-2™ (Fisher & Paykel Healthcare, Auckland, New Zealand), consisting of a flow generator up to 60 Lt/min, an air-oxygen blender and an auto-fill heated chamber that allows for adjustment of FiO2 from 21% to near 100%. The gas mixture at 34 to 37°C was delivered via a single-limb heated breathing tube to the patient via Optiflow™ nasal cannula (Fisher & Paykel, Auckland, New Zealand).
Demographic data, immunocompromise origin, Respiratory Rate (RR), Heart Rate (HR), Mean Arterial Pressure (MAP), oxygen saturation (SpO2) and PaO2:FiO2 ratio were extracted previously and recorded during the first 24 hours of HFNC connection. It was also registered the need for higher ventilatory support after HFNC, orotracheal intubation (OTI) requirement and 90 days out-hospital mortality. For descriptive statistics, demographic data were expressed in median [IQR] and absolute and relative frequencies, and for the pre and post-HFNC connection, analysis data were expressed in means ± standard deviation. For the pre and post-association with HFNC of the variables HR, RR, MAP, SpO2 and PaO2:FiO2 ratio, a paired T-Student test was performed, assuming normal distribution by a central theory of the limit. A level of significance of 95% was considered. The Stata 15TM statistical package was used for statistical analysis (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC.).
Data were extracted from the ICU prospective registry (RUCI), which contained anonymous epidemiological and clinical data of ICU admitted patients, and an Informed Consent form has been signed. RUCI database has been approved by our IRB and local Ethics Committee since 2012 (2012-53).
3. RESULTS
25 adult immunocompromised patients met inclusion criteria with a median age of 64 years, with 68% males, median APACHE II 15 [11-19] points, median PaO2:FiO2 ratio 219. 84% had a cancer diagnosis, while the remaining 16% had a pharmacological or human immunodeficiency virus (HIV). 76% of the sample was admitted for ARF-support, 58% of them with pneumonia diagnosis. HFNC connection reasons were hypoxemic ARF for 64%, hypercapnic ARF for 4%, to facilitate IMV weaning for 4%, to facilitate NIV weaning for 20% and NIV-intolerance for 8%. Patients had a median of 6 days HFNC connection and the setting used included a flow of 49 Lt/min and a T° of 34.7 ºC (Table 1).
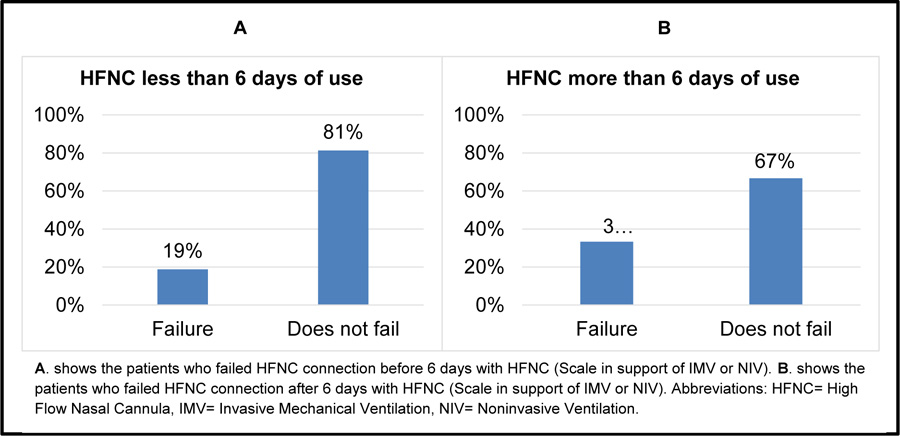
After the HFNC connection, 64% of the subjects used conventional oxygen therapy, 12% did not need any respiratory support (FiO2 21%), 8% of the subjects had to be connected to NIV, and 16% to IMV. Patients required a higher ventilatory support (NIV, IMV) post HFNC connection, and 12% died due to maintained hypoxemia, 4% respiratory acidosis, 4% increased WOB and 4% HFNC-intolerance. The mean RR before HFNC connection was 25±22 breaths/min, and after HFNC, the mean was 22±4 breaths/min (p=0.02). HR mean before HFNC connection was 96±22 beats/min, and after, it was 86±15 beats/min (p=0.008). Previous MAP mean was 86±15 mmHg, and after, it was 80±12 mmHg (p=0.09); previous SpO2 mean was 93±5%, and after, it was 95±4% (p=0.13); while previous PaO2:FiO2 ratio mean was 219±66, and after, it was 324±110 (p=0.52). (Table 2) 64% of patients were connected for less than 6 days to HFNC, of whom, 81% did not require further ventilatory support. 36% were connected for over 6 days and 67% of them did not require higher ventilatory support (Fig. 3).
| - | Pre HFNC | Post HFNC | p |
|---|---|---|---|
| PaO2:FiO2 ratio, Means ± SD | 218.9±66.1 | 323.8±110.4 | 0.52 |
| RR, breaths/min, Means ± SD | 25±22.3 | 21.7±4.1 | 0.02 |
| HR, beats/min, Means ± SD | 96.5±22 | 85.9±14.8 | 0.008 |
| MAP, mmHg, Means ± SD | 85.7±15.3 | 79.7±11.8 | 0.09 |
| SpO2, %, Means ± SD | 93±5 | 95±4 | 0.13 |
Finally, 28% of subjects died during hospitalization, 8% of which due to a limitation of therapeutic effort, and 32% died within 90 days after discharge. The global mortality rate of the 25 immunocompromised patients included in this study was 60%.
4. DISCUSSION
Several studies have described HFNC physiological effects that explain the observed clinical improvements, such as reduced WOB [8, 9, 12-14, 16]. In this study, we found a significant reduction in RR and HR post-HFNC connection in immunocom- promised patients with ARF, similar to the RCT results of Lemiale (2017) [19], Xiaofeng Ou (2017) [18] and Frat (2015) [8], where HFNC reduced in a meaningful way RR and HR (both taken as surrogate variables), describing an improvement in WOB. Regarding oxygenation, HFNC seems not to cause changes despite being a high flow oxygen therapy system. In this study, we found that PaO2:FiO2 ratio tend to improve with a significant clinical change (an increase of 100 points) but not statistically significant, similarly to the results observed by Adda (2008) [20-22] and Xiaofeng Ou (2017) [20].
Over the last decade, HFNC has emerged as an effective alternative treatment to conventional oxygen therapy and NIV. Our studied population showed an increase in intubation and the use of IMV when HFNC was prolonged. Similar results have been described by Lee HY (2015) [23] that a delay in intubation increases the risk of death. Results of this study showed that patients with increased respiratory support before 6 days of connection needed NIV, while those who remained on HFNC for more than 6 days died and required connection with IMV. These results indicate that HFNC although maintains good oxygenation, cannot provide respiratory support without respiratory monitoring. In fact, all these patients required OTI; however, none of them died while on IMV. These results are consistent with the evidence that has shown that the survival of this subgroup of patients with IMV has increased from 10% to 40% [21] as a result of the advances in treatment and IMV strategies [22].
The mortality rate in this study was 60%, similar to that described by Lee HY (2015) [23], who showed a mortality rate of 62%, which allows us to conclude indirectly that due to ominous prognosis and diagnostic variability, immunocom- promised patients maintain a high mortality rate independent of the ventilatory strategy used. New evidence supports our results, suggesting that independently of the chosen respiratory strategy, mortality in immunocompromised patients remains high and this could be related to a delay in ICU admission. Etiology determination at hospital admission or the correct respiratory support for these specific patients are needed [24]. Hui-Bin Huang (2017) reported in a review and meta-analysis that HFNC reduces intubation and mortality rates but better quality studies and RCTs are needed for confirmation of this result [25].
Due to being a retrospective study with data from a single patient registry, our study possessed some limitations such as selection bias, excluding patients with known outcomes, information bias in recollection, registration and missing data. As we did not perform a multivariate analysis, we could not adjust the results for covariates like age, type and immuno- suppression status or prognosis, so this would be desirable in future studies. The results of this study are poorly generalizable and only are valid for the immunocompromised population.
CONCLUSION
Our results show that in immunocompromised HFNC-supported patients, RR and HR decrease. In-hospital mortality in ARF immunosuppressed patients was high even though respiratory support was used. HFNC appears to be an attractive alternative in immunocompromised patients with ARF and often with limited therapeutic effort. Major studies are necessarily required to define the role of HFNC in immunocompromised ARF supported patients.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by Clinica Alemana, Santiago IRB and local Ethics Committee (2012-53).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
All patients participated on a voluntary basis and gave their informed consent.
STANDARDS OF REPORTING
STROBE guidelines and methodologies were followed in this study.
AVAILABILITY OF DATA AND MATERIALS
For this study, data were extracted from the ICU prospective registry (RUCI), which contained anonymous epidemiological and clinical data of ICU admitted patients.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


