All published articles of this journal are available on ScienceDirect.
Tiotropium Bromide: An Update
Abstract
Tiotropium bromide is a once-daily inhaled anticholinergic bronchodilator. It works by blocking the muscarinic receptors in airway smooth muscle. Tiotropium has a wide therapeutic margin, due to its poor gastrointestinal absorption and its very low systemic bioavailability. The drug is mainly indicated in COPD patients. Clinically relevant outcomes such as significant improvements in spirometry, hyperinflation, dyspnea, heath status, acute exacerbations and mortality have been consistently observed in tiotropium clinical trials, and the drug has been shown to reduce the risk of mortality due to cardiac-vascular disease and respiratory failure. The main side effect reported is dryness of the mouth. Some subgroups of asthmatics also seem to respond to anticholinergic drugs: among them, those with the Arg/Arg genotype for the β2-adrenergic receptor and those with a high percentage of neutrophils in sputum.
INTRODUCTION
Chronic obstructive lung disease (COPD) is a widespread chronic disease that mainly affects long-term smokers. As its name indicates, it is characterized by airway irreversibility. To date, the results of treatment for this disease have been relatively disappointing and no drug has proved able to reverse, or even to check, its development. In this paper we review the properties, pharmacological and clinical effects of tiotropium bromide, the latest drug to come on the market for treatment of chronic lung obstruction.
DRUG CHARACTERISTICS
Tiotropium bromide is a synthetic quaternary anticholinergic agent approved for maintenance therapy in stable COPD. It has two important properties: it is functionally selective for specific muscarinic receptors that mediate airway smooth-muscle contraction, and it has a long duration of action, making it well suited for once-daily dosing. Tiotropium works by blocking the muscarinic receptors for the neurotransmitter acetylcholine (Ach), which is released from cholinergic nerve endings in the airways. As tiotropium bromide is electrically charged, it is not absorbed by the gastrointestinal tract and does not pass the blood-brain barrier. This means that it does not have characteristic side effects of anticholinergic agents, particularly those affecting the central nervous system.
MUSCARINIC RECEPTORS IN THE AIRWAYS AND TIOTROPIUM BROMIDE
Three muscarinic receptor subtypes have been found in human airways, with different functions: M1, M2 and M3 [1]. Autoradiographic mapping has shown that these receptors are mainly localized in the smooth muscle of all airways, although they reach their highest density in the proximal airways and in submucosal glands [2].
M1 receptors are localized in parasympathetic ganglia in the airway, where they function as regulators of ganglionic transmission (Fig. 1). Preganglionic nerves release Ach, which acts on th nicotinic receptors of the ganglionic cells to activate postganglionic nerves. M1 receptors facilitate neurotransmission through these ganglia, thereby enhancing the cholinergic bronchoconstrictor reflex [3]. Therefore, blocking M1 receptors will be beneficial in COPD patients.
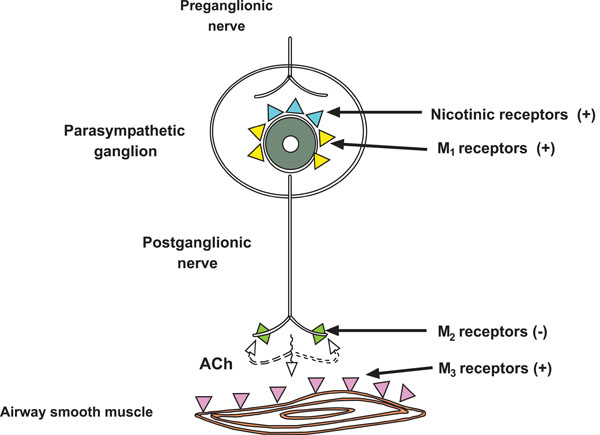
Muscarinic receptor subtypes in airways. M1 are localized to parasympathetic ganglia. M2 on postganglionic cholinergic nerve terminals inhibit the release of Ach. M3 that constrict airway smooth muscle. Adapted from Barnes PJ [15].
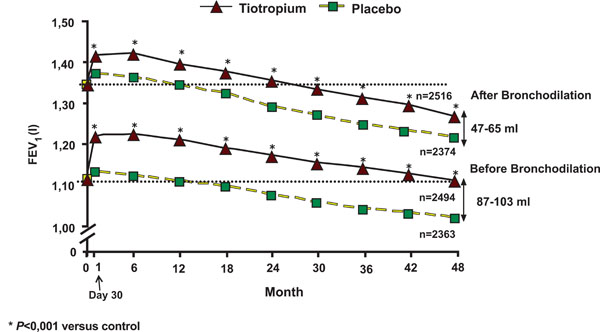
Mean forced expiratory volume in 1 second before and after bronchodilation from day 30 to the end of the study. From Tashkin et al. [29].
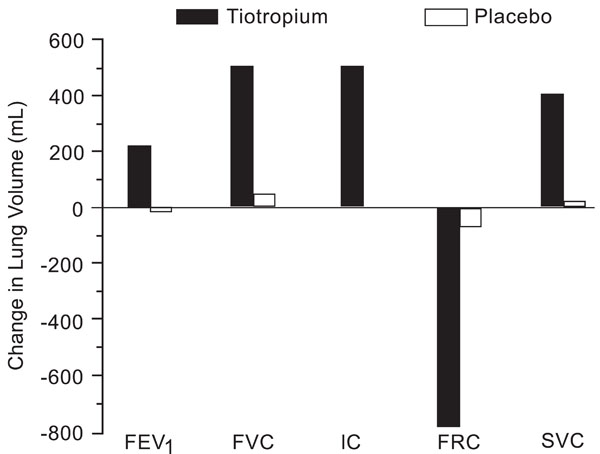
Changes in lung volumes and spirometry following treatment with tiotropium or placebo after 4 weeks. All differences between treatments were significant (p<0.01). From Celli et al. [36].
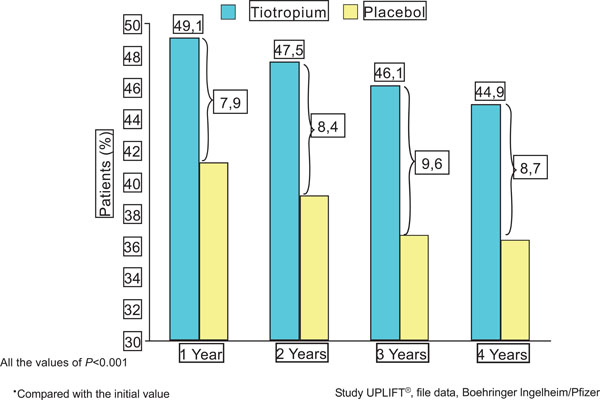
Tiotropium significantly increases the percentage of patients with an improvement ≥4 units in the total score of the SRGQ*. File data, Boehringer Ingelheim/Pfizer.

Kaplan- Meier curves show the cumulative incidence estimate of the probability of COPD exacerbation at day 1470. All patients who received at least one dose of a study drug were included in the analysis. From Tashkin et al. [29].
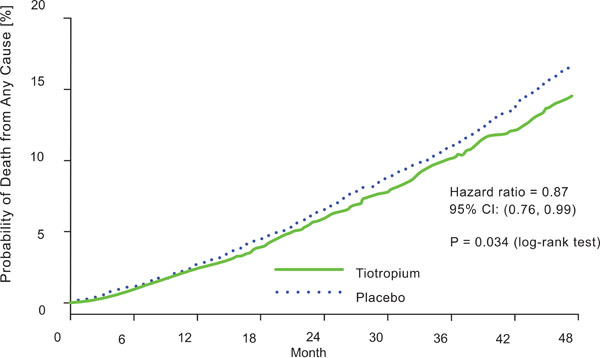
Kaplan-Meier estimates of the probability of COPD death from any cause. From Tashkin et al. [29].

Mean forced vital capacity in before and after bronchodilation from day 30 to the end of the study. From Tashkin et al. [29].

Spearman rank correlations (rs) between the change in forced expiratory volume in one-second after 4 weeks of tiotropium bromide administration and (a) eosinophil percentages or (b) neutrophil percentages in induced sputum. Eosinophil and neutrophil percentages were determined prior tiotropium bromide administration. In all subjects (a) rs =0.669. p=0.003 and (b) rs =0.540. p=0.025. In smokers (a) rs =0.706. p=0.034 and (b) rs =0.361. p=0.339. In nonsmokers (a) rs =0.762. p=0.028 and (b) rs =0.762. p=0.028. ○ smokers; ● nonsmokers. From Iwamoto et al. [67].
M2 receptors are located on cholinergic nerve endings and act as autoreceptors which inhibit acetylcholine release from those nerve terminals [4], thereby providing feedback inhibition (Fig. 1). Drugs block M2, increasing Ach release, and thus increasing bronchoconstrictor responses.
M3 receptors are expressed in the smooth muscle of all airways (Fig. 1). The bronchoconstrictor response to cholinergic nerve stimulation and cholinergic agonists is mediated by M3 [5]. These receptors also mediate mucus secretion in response to cholinergic agonists [6]. For this reason, blockade of M3 receptors is the main objective of anticholinergic therapy in COPD. The major side effects of anticholinergic drugs – dry mouth, glaucoma, and urinary retention – are all mediated by M3 receptors, so their frequency cannot be reduced.
Therefore, optimal inhibition of parasympathetic activity may be achieved by selective antagonism of the M3 and M3 receptors, sparing the M3 receptors. Tiotropium bromide dissociates more slowly from M1 and M3 receptors than M2 receptors and for this reason has kinetic selectivity [7]; because of its sustained activity, once-daily administration is possible. Tiotropium bromide binds to muscarinic receptors with high affinity and is approximately 10 times more potent than ipratropium bromide in binding to human lung muscarinic receptors [8]. Ipratropium bromide and oxitropium bromide are nonselective blockers; consequently, they block M2 receptors, thereby increasing Ach release at nerve endings, which may reduce the degree of blockade or the duration of action [4].
It has been demonstrated that the slow dissociation of tiotropium bromide from target protein is a key factor in its long duration effect [7]. Though the two compounds have similar pharmacokinetic profiles, tiotropium’s duration of action is more than 24-h, while ipratropium action lasts only six hours [9]. Studies using recombinant receptors have shown that tiotropium has a much slower rate of dissociation from M3 muscarinic receptors than ipratropium [10], and conclude that this feature determines the difference in duration of action between the drugs [7].
The approved dose of tiotropium is 18μg qd, preferably administered in the morning [11]. As bronchodilation tends to increase over the first week of daily administration, this dose is best used on a regular daily basis. Since tiotropium may take several hours to reach its peak effect, it is not recommended for rapid relief of dyspnea. A bronchodilator reversibility test prior to prescription does not predict its long-term benefits [12].
Tiotropium has been shown to reduce the frequency of exacerbations, but its effect on airway inflammation is unclear. A placebo-control trial investigating its effect on inflammation parameters such as sputum and serum cytokines found an association with a reduced frequency of exacerbation, but there was no difference in airway or systemic inflammation [13]. A recent study [14] proposes that tiotropium may have a beneficial influence on airway remodeling in chronic airway diseases through its antiproliferative effects on fibroblasts and myofibroblasts.
THE ROLE OF TIOTROPIUM IN THE MANAGEMENT OF COPD
There is some evidence that the cholinergic tone of the airways is increased in patients with COPD [16]. In fact vagal cholinergic tone is the main reversible element of airway obstruction in COPD, and its effects are exaggerated by geometric factors due to narrowed airways, because airway resistance is inversely proportional to the fourth power of the airway radius [17].
Tiotropium significantly improves bronchodilation, hyperinflation, dyspnea, health status, exacerbations and mortality in COPD patients, but it does not decrease the rate of FEV1 decline in COPD.
BRONCHODILATION
The degree of acute improvement in spirometry indices after bronchodilator inhalation varies among COPD patients, and depends on the type and dose of bronchodilator as well as the timing of administration. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as a disease characterized by a partially reversible airflow limitation [18]. Patients with COPD are commonly thought to present a lower acute bronchodilator response than asthmatics, although a high percentage of patients with COPD respond to high doses of β2 adrenergic receptors [19]. Moreover, more than half of COPD patients who do not significantly respond to β2 adrenergic receptors have positive bronchodilator tests when high doses of inhaled anticholinergics are administered [20]. A recent trial in a large cohort of patients with moderate-to-very-severe COPD found significant increases in lung function in most patients who received 80 µg ipratropium followed by 400 µg salbutamol. The percentage of patients considered as responders differed depending upon the criterion applied [21]. Almost two-thirds (65.6%) of patients met at least one common criterion for FEV1 responsiveness following acute administration of bronchodilators [22].
FEV1 has been shown to rise slowly after tiotropium administration, reaching a peak between 1 to 3 h after the inhalation. The peak response is followed by a plateau significantly higher than placebo, lasting up to 32 h [9].
Tiotropium has shown an effective bronchodilation effect following single administrations of doses of between 10 and 80 mg [9]. All subsequent studies have used a daily dose of 18 mg. After drug withdrawal, patients’ FEV1 gradually returned to baseline levels over a period of approximately 3 weeks, indicating that the drug exerted a prolonged effect [23].
Repeated daily use of tiotropium offers persistent bronchodilation. The FEV1 24 hours post-dose (and before the next dose), its “trough” value, progressively increases over the first few days of regular administration. Van Noord et al. [24] found that the trough FEV1 increased by 0.19 L (18% above the initial baseline) after 8 days of daily administration, most of this increase having occurred by the second day. Trough FVC continued to rise daily during the first week and had increased by 0.67 L (27% above its initial baseline) by day 8.
The results of large long-term trials indicate that tiotropium administered once daily raises the trough FEV1 by 0.1 to 0.15 L, and raises the peak FEV1 by a further 0.15 to 0.20 L. In both cases, its effect is significantly higher than that of either ipratropium administered four times daily or salmeterol administered twice daily. No loss of efficacy was seen over the course of one year of regular treatment with tiotropium. Therefore, it appears that the difference at the end of the trial may be due, at least to some extent, to the fact that the response to tiotropium was maintained, whereas the response to salmeterol decreased over the course of the study, suggesting the development of tachyphylaxis [25-28]
In the UPLIFT study [29], the mean FEV1 value in the tiotropium group after the bronchodilation test showed a significant improvement compared with the placebo group, which was maintained throughout the study. This improvement ranged from 47 to 65 ml after bronchodilation (p<0.001) (Fig. 2). The tiotropium group also showed a significant improvement in the FEV1 before bronchodilation, ranging from 87 to 103 ml (p< 0.001) (Fig. 2). But in patients with stable COPD there was little difference in bronchodilation when adding higher than conventional doses of salbutamol or ipratropium bromide to tiotropium. This is an interesting finding, especially for COPD patients suffering from cardiovascular co-morbidities [30]. On the other hand, in patients with COPD the addition of formoterol once or twice daily to tiotropium once daily improves airflow obstruction, resting hyperinflation, and the use of rescue salbutamol [31]. No such improvement was found when we added salmeterol to tiotropium [32].
HYPERINFLATION
In COPD patients, hyperinflation is reflected by a significant increase in static lung volumes. Lung volume changes correlate better with impairment of patient functional capabilities than airflow limitations [33]. The systematic use of tiotropium may produce a significant reduction in lung inflation.
The inspiratory capacity (IC) is a parameter that can be measured by spirometry and its increase indirectly reflects a decrease in hyperinflation [34, 35]. In patients with COPD receiving 4-week treatment with tiotropium versus placebo the functional residual capacity was substantially reduced and IC was increased in the treated group, indicating a reduction in hyperinflation that did not occur in the placebo group [36] (Fig. 3). Another clinical trial found that the reduction in hyperinflation led to an increase in the tidal volume during constant work-rate cycle ergometry, with an improvement in endurance time of approximately 21%. The dyspnea index also improved after six weeks of treatment [37].
Recently, Gelb et al. studied the protection of tiotropium against dynamic hyperinflation in COPD phenotypes. Emphysema was evaluated by high-resolution thin-section lung scan. Spirometry (including inspiratory capacity) was measured before and immediately after metronome-paced hyperventilation to induce dynamic hyperinflation. Tiotropium increased IC in moderate-severe COPD, independently of the severity of lung emphysema. Despite bronchodilation and lower resting lung volume, tiotropium did not decrease the induced dynamic hyperinflation, which was also independent of underlying emphysema [38].
DYSPNEA
In COPD patients, dyspnea correlates more closely with hyperinflation than FEV1 [33, 34]. The effect of tiotropium on this symptom has been measured by Mahler’s transition dyspnea index (TDI) [39] for the dyspnea incurred by activities of daily living in COPD. The improvement in TDI in patients receiving tiotropium has been shown to be statistically higher than placebo and ipratropium at all time points over a 12-month period and tends to increase as treatment progresses [25, 26]. In the studies comparing salmeterol versus tiotropium, the improvement in TDI with tiotropium was greater than with salmeterol [27-28] although this difference only reached statistical significance in one study [27]. COPD symptoms can be measured indirectly with the use of a rescue short-acting β2-agonist. A treatment that effectively reduces symptoms should also reduce the need for rescue medication; studies have shown that tiotropium performs better than both placebo and ipratropium [25, 26].
Pulmonary rehabilitation has been shown to improve exercise tolerance as well as dyspnea [40, 41]. In a placebo-controlled trial, Casaburi et al. [42] tested the hypothesis that improvement in ventilatory mechanics resulting from tiotropium treatment may enhance patients’ training of muscles involved in deambulation and therefore increase exercise tolerance and the benefits of pulmonary rehabilitation. In that study, tiotropium in combination with pulmonary rehabilitation improved endurance of a constant work-rate treadmill task and produced clinically significant improvements in dyspnea and health status compared with pulmonary rehabilitation alone. In addition, improvements with tiotropium were sustained for three months after completion of pulmonary rehabilitation [42].
HEALTH STATUS
The effects of tiotropium on health status have been measured in several studies. The St. George’s Respiratory Questionnaire score, measured in long-term studies, improved significantly with tiotropium treatment compared to baseline, placebo [25, 27, 28] or ipratropium [26], but not compared to sameterol [27, 28].
In the one-year tiotropium trials using The Medical Outcomes Study Short Form-36, a generic health status instrument, the domains of role-physical and physical health summary scores significantly improved in the tiotropium group compared to the control groups [25-28].
In the UPLIFT study [29], 2478 patients treated with tiotropium and 2337 patients receiving placebo were included.
The treated group showed significant differences at all time points for the mean absolute change in the SGRQ total score, although the differences were not considered to be clinically significant. However, a higher proportion of patients in the tiotropium group compared to the placebo group had an improvement of four points or more in the SGRQ total scores from baseline at the end of the fourth year of treatment (Fig. 4).
ACUTE EXACERBATIONS
Late stage COPD is characterized by increased frequency and severity of exacerbations, which account for most of the condition’s economic burden. Exacerbations are associated with worse prognosis and increased mortality, especially those that require hospital admission [43].
The effect of tiotropium treatment on the incidence of acute exacerbations has been considered as a secondary outcome in several studies. Modifying the criteria of Anthonisen et al. [44], exacerbations in the tiotropium studies were defined as an increase in two or more respiratory symptoms for at least three days. Casaburi et al. [25] enrolled 921 patients with stable COPD in two identical randomized one-year double-blind placebo-control studies, and found significantly fewer exacerbations in the tiotropium group than in the placebo group (20% reduction). There was a significant increase in the time to first exacerbations in the tiotropium group compared to placebo. The proportion of patients experiencing at least one COPD exacerbation was lower in the tiotropium group (36%) than in the placebo group (42%) (14% reduction, p< 0.05). In addition, significantly fewer hospitalizations were associated with exacerbations in the tiotropium cohort compared to the placebo group (47% reduction). The proportion of patients hospitalized for exacerbations was lower in the tiotropium group than in the placebo group (41% reduction, p > 0.05).
Another trial [28] recorded exacerbations in 1207 patients with COPD during six months’ treatment with tiotropium, salmeterol, or matching placebos. Compared with placebo, tiotropium, but not salmeterol, was associated with a delay in the onset of the first exacerbation. Fewer COPD exacerbations/patient-year occurred in the tiotropium group than in the placebo group (p<0.05); there were no differences between the salmeterol and the placebo groups. The tiotropium group showed 0.10 hospital admissions per patient-year per COPD exacerbation, compared with rates of 0.17 for salmeterol and 0.15 for placebo (non-significant differences).
The INSPIRE study [45] was the first large-scale trial to evaluate the impact of two different treatment approaches – tiotropium or the combination using salmeterol and fluticasone propionate – on COPD exacerbations over a 2-year period. Methodological issues need to be considered when interpreting these results (see the mortality section). The estimated overall rates of exacerbations were 1.28 per year with the combination and 1.32 per year for tiotropium; the ratio (0.967) indicated that there was no difference between the rates. The incidence of exacerbations requiring hospitalizations was 16% for salmeterol and fluticasone propionate, and 13% for tiotropium.
Two meta-analyses evaluating the effectiveness of tiotropium bromide compared to placebo, ipratropium bromide or a long-acting beta-agonist for the treatment of stable COPD patients showed that tiotropium notably reduced the exacerbations and related hospitalizations compared with placebo, but no statistical differences were found with respect to the long-acting beta2-agonist (LABA) [46, 47].
Two recent trials have evaluated the potential benefits of associating tiotropium with LABA and inhaled corticosteroids. Aaron et al. [32] report the results of the first clinical study in moderate to severe COPD after one year of follow-up in three groups of patients receiving tiotropium plus placebo, tiotropium plus salmeterol and tiotropium plus fluticasone-salmeterol. Similar proportions of patients experienced an exacerbation across all three groups, but those who received tiotropium plus fluticasone-salmeterol presented lower levels of COPD and all-cause hospitalizations compared with those who received tiotropium plus placebo. The addition of salmeterol to tiotropium had no effect on hospitalization rates compared to tiotropium alone. A limitation of the study is the sizable proportion of patients (approximately 40%) who did not receive the assigned treatment throughout the study.
In the UPLIFT study, exacerbations were considered a secondary outcome [29]. The trial compared four years of therapy with either tiotropium or placebo in patients with COPD who were permitted to use all respiratory medications except inhaled anticholinergic drugs. Tiotropium was associated with a 14% reduction in the mean number of exacerbation (p>0.001). The drug was associated with a significant delay in the time to the first hospitalization for an exacerbation (Fig. 5). Tiotropium was also associated with a significant delay in the time to the first exacerbation: a median of 16.7 months (95% CI: 14.9 to 17.9) compared with one of 12.5 months (95% CI: 11.5 to 13.8) in the placebo group.
MORTALITY
In patients with COPD, all-cause mortality significantly decreases after smoking cessation. Mortality did not significantly differ in patients receiving ipratropium bromide or placebo [48]. Comparing four groups of patients receiving almeterol, fluticasone propionate, both drugs or placebo with death from any cause as the primary outcome, the TORCH study [49] found all-cause mortality rates to be similar in the four arms.
Mortality was a secondary outcome in a 104-week long trial [45] comparing the clinical efficacy of treatment with a fixed combination of salmeterol/fluticasone propionate and tiotropium bromide. Mortality was significantly lower in the salmeterol/fluticasone propionate group: 3% of patients in this group died, compared with 6% in the tiotropium group (p=0.032). However, methodological issues need to be considered when interpreting these results [50]. In this study [45] the design imposed discontinuation of inhaled corticosteroids followed by a 2-week run-in of oral prednisolone and salmeterol prior to randomization, which may have affected outcomes [51]. Other effects were likely due to the abrupt interruption of the run-in regimen. Another methodological problem that hampers the interpretation of the results is the incomplete follow-up of patients, which affects the intent-to-treat analysis.
Two recent trials have shown that tiotropium reduces mortality. A longitudinal, population-based cohort study was conducted to compare the effect of tiotropium use and LABA use/OK? on all-cause mortality in patients with COPD [52]. Patients included individuals aged 65 or older discharged from hospital with a diagnosis of COPD. The effect of receiving a prescription for tiotropium compared to a LABA on all-cause mortality at 180 days post-hospital discharge was eliminated by controlling the potential confounders. Data from 7218 eligible patients were analyzed. Of these, 1046 (14.5%) died in the follow-up period. Patients who received tiotropium were 20% less likely to die than those receiving a LABA (hazard ratio 0.80, 95% CI 0.70 to 0.93).
In the UPLIFT study [29], vital-status information was available after a 45 month follow-up for 98% of patients in the tiotropium and for 97% in the placebo group (including patients who discontinued treatment). Tiotropium significantly reduced mortality. Nine hundred and twenty-one patients died: 14.4% in the tiotropium group and 16.3% in the placebo group (hazard ratio, 0.87; 95% CI: 0.76 to 0.99) (Fig. 6).
RATE OF DECLINE IN LUNG FUNCTION
With the exception of smoking cessation, no intervention has been shown to decrease the rate of FEV1 decline in COPD [53]. Two identical 1-year, randomized, double blind, double-dummy studies involving 535 patients with COPD showed a sustained improvement in lung function. Tiotropium (18 µg once daily) increased trough FEV1 by 120 ml by the end of the trial, whereas treatment with ipratropium (40 µg 4 times daily) was associated with a 30-ml reduction in trough FEV1 [26]. A post hoc analysis was performed using data from 921 patients [54]. The change in trough FEV1 was –12.4 ml/yr in the tiotropium group compared with –58.0 ml/yr in the placebo group (p = 0.005). Similar results were observed in both former and current smokers. In former smokers, the change in trough FEV1 was –17.0 ml/yr with tiotropium and –67.9 ml/yr with placebo (p = 0.011), whereas in smokers the changes in trough FEV1 were –3.8 ml/yr and –40.5 ml/yr respectively (p = 0.19). The lack of statistically significant differences for FEV1 in smokers may be due to the small number of patients considered when the analysis was stratified by smoking status.
To determine whether tiotropium slows the decrease in lung function over time, a 4-year study [29] was performed. Therapy other than inhaled anticholinergic drugs was allowed in accordance with current COPD guidelines. There were no significant differences between study groups in the rate of decline in the mean values for FEV1 and FVC either before or after bronchodilation from day 30 to the end of study (Fig. 7). In the tiotropium group, the mean values for FVC and FEV1 before and after bronchodilation showed significant improvements in FEV1 compared to the placebo group, ranging from 87 to 103 ml before bronchodilation and from 47 to 65 ml afterwards. The rate of decline in subgroups analyses showed no significant differences according to age, gender, smoking status, Gold stage and reversibility. In a post hoc analysis, between-group differences in the rate of decline in post-bronchodilator FEV1 were observed, with tiotropium again performing better than placebo (40 ± 3 ml in the tiotropium group and 47 ± 3 in the placebo group, p = 0.046) in the subgroup of 1554 patients who were not receiving either inhaled corticosteroids or LABA at baseline.
Compared with results of previous long-term studies, the rate of decline in FEV1 observed in the UPLIFT study was numerically lower than those reported previously [55-58] and similar to those reported for healthy nonsmokers and sustained quitters with mild-to-moderate COPD [53] (Table 1).
Result of Long-Term Studies in the Rate of Decline in FEV1
| Study | Active Smoker | FEV1 % | Drug of Study | Rate of Decline FEV1 (mL/y) | ||
|---|---|---|---|---|---|---|
| Dug | Only Placebo | Placebo* | ||||
| EUROSCOP | 100% | ≈ 79% | Budesonide | 57 | 69 | - |
| ISOLDE | 36-39% | ≈ 50% | Fluticasone | 50 | 59 | - |
| LHSII | 90% | ≈ 68% | Triamcinolone | 44 | 47 | - |
| BRONCUS | 41-51% | ≈ 57% | NAC | 54 | 47 | - |
| TORCH | 43% | ≈ 48% | S/F/SF | 42/42/39 | 55 | - |
| UPLIFT | 30% | ≈ 47% | Tiotropium | 40 | - | 42 |
* All respiratory medications permitted throughout the trial, other than inhaled anticholinergics.
The current treatment for COPD may affect the decline in lung function and may have a ceiling effect, with the result that no further improvements are seen in the absence of an intervention that repairs or regenerates lung tissue [29].
SIDE EFFECTS OF TIOTROPIUM
Tiotropium has a wide therapeutic margin due to its poor gastrointestinal absorption and thus has very low systemic bioavailability. To date, no unfavorable interactions between tiotropium and other drugs have been reported.
Kesten et al. [59] conducted a pooled analysis of adverse event data on 7819 patients with COPD from 19 randomized, double blind, placebo-control trials with tiotropium. Inherently serious cardiac conditions such as cardiovascular mortality (RR: 057; 95% CI: 0.26 to 1.26), cardiac arrest (RR: 0.90; 95% CI: 0.26 to 3.15), and myocardial infarction (RR: 0.74; 95% CI: 0.26 to 2.07) were not more frequent among patients receiving tiotropium than patients receiving placebo. Among heart rate and rhythm disorders, the relative risk of tachycardia (excluding ventricular tachycardia and fibrillation) was 1.68 (95% CI: 0.69 to 4.11) for any degree of the condition and 1.16 (95% CI: 0.33 to 0.43) for serious tachycardia. The relative risk of left-heart failure among patients receiving tiotropium compared with patients receiving placebo was 0.46 (95% CI: 0.21 to 1.00). There was no increase in total and serious events due to ischemic heart disease.
For GI disorders, there was a higher risk of dry mouth in patients receiving tiotropium (RR, 3.60; 95% CI, 2.56 to 5.05), but none was classified as serious. The relative risk of dysphagia was 5.91 (95% CI, 0.60 to 58.31). There was no increased risk of abdominal pain, constipation, dyspepsia, or nausea associated to tiotropium use.
Among renal and urinary disorders, the relative risk of urinary retention in patients receiving tiotropium compared to placebo was 10.93 (95% CI: 1.26 to 94.88). Although the relative risk of serious prostatic disorders was 5.32 (95% CI: 0.59 to 48.33), the analysis of all selected events showed no association of tiotropium (RR: 1.04; 95% CI: 0.46 to 2.35).
As for skin disorders, the relative risk of pruritus in tiotropium users was 1.61 (95% CI: 0.68 to 3.82). There was no increase in the risk of serious events among these disorders. An analysis of other selected events indicated no increased risk of glaucoma, other visual disturbances, chest pain, edema, fungal infections, musculoskeletal and connective tissue disorders, central nervous system (CNS) or psychiatric disorders.
In UPLIFT study, for all-cause mortality, the relative risk in patients receiving tiotropium compared to patients receiving placebo was 0.76 (95% CI: 0.50 to 1.16). The relative risk of cardiovascular mortality was 0.57 (95% CI: 0.26 to 1.26) and of respiratory mortality was 0.71 (95% CI: 0.29 to 1.74).
A recent publication [60] has raised concerns about the safety of tiotropium bromide. Assessing the results of a meta-analysis of 17 previously conducted clinical trials, Singh et al. suggested an association between inhaled anticholinergics and an increased risk of cardiovascular death and myocardial infarction and stroke. However, the methodology of Singh et al.’s study raises several important concerns. First, placebo controlled trials were considered together with active controlled trials, implicitly assuming that the comparator drug is interchangeable with a placebo. Second, the analysis does not take into account the fact that in most trials more patients in the placebo group dropped out than patients taking active medication, and that they were followed for briefer periods of time, during which adverse events were reported. Third, most of the evidence in the analysis is from The Lung Health Study [61], in which most of the cardiovascular deaths occurred among patients who were not using their inhalers; clearly, these deaths could not be due to a medication they were not taking. Finally, the dataset analyzed appears to have an important methodological flaw that potentially invalidates the findings: it appears to include two studies twice, thus double-counting more than one thousand patients.
Two recent studies [29, 62] found that tiotropium was associated with reduced respiratory and overall mortality and was not associated with increased cardiac mortality. In the UPLIFT study [29], respiratory failure developed in 88 patients in the tiotropium group and in 120 in the placebo group (RR: 0.67; 95% CI: 0.51 to 0.89); myocardial infarction developed in 67 patients in the tiotropium group and in 85 in the placebo group (relative risk, 0.73; 95% CI: 0.53 to 1.00) and stroke developed in 82 patients in the tiotropium group and 80 in the placebo group (RR: 0.95; 95% CI: 0.70 to 1.29). In summary, tiotropium seems to reduce the risk of mortality due to cardiac and vascular causes. Furthermore, it does not increase the risk of stroke and it may decrease the risk of myocardial infarction.
THE ROLE OF TIOTROPIUM IN THE MANAGEMENT OF ASTHMA
Anticholonergic drugs inhibit reflex cholinergic bronchoconstriction but are unable to significantly block the direct effects of inflammatory mediators such as histamine, kinins and leukotrienes on bronchial smooth muscle. This is why they are less effective bronchodilators in asthma than β2-agonists. However, in a study of 12 atopic male asthmatic patients, tiotropium provided significant protection against a methacoline challenge for 48 h [63].
Some subgroups of asthmatics seem to respond better to anticholinergics. Some authors aiming to identify these subgroups have suggested the following clinical profile: patients with nocturnal symptoms, chronic asthma showing concurrent fixed airway obstruction, intrinsic asthma with longer duration of disease and non-atopic asthma [63, 64]. Today, there are three trials that identify patients with asthma who respond to tiotropium:
- A genotype-stratified study revealed a greater bronchoprotective effect of anticholinergic agents in asthmatic patients with the Arg/Arg genotype of the β2-adrenergic receptor [65].
- COPD and asthma are common and can occur in the same patient. In this case, chronic use of tiotropium achieves spirometric improvements with symptomatic benefits, as seen by reduced need for rescue medication [66].
- Iwamoto et al. [67] showed an association between responsiveness to tiotropium bromide and the presence of different inflammatory cells in the induced sputum of patients with severe persistent asthma treated with moderate-to-high doses of inhaled corticosteroids and other anti-asthma agents. The percentages of eosinophils in induced sputum were inversely correlated with ΔFEV1, whereas the proportions of neutrophils were positively correlated with ΔFEV1. Comparable results from nonsmoking asthmatics indicate a rationale for using tiotropium bromide to treat severe patients/with a noneosinophilic sputum profile (Fig. 8).


