All published articles of this journal are available on ScienceDirect.
Unusual Bacterial Infections and the Pleura
Abstract
Rickettsiosis, Q fever, tularemia, and anthrax are all bacterial diseases that can affect the pleura. Rocky Mountain Spotted Fever (RMSF) and Mediterranean Spotted Fever (MSF) are caused by Rickettsia rickettsii and Rickettsia conorii, respectively. Pleural fluid from a patient with MSF had a neutrophil-predominant exudate. Coxiellaburnetii is the causative agent of Q fever. Of the two cases described in the literature, one was an exudate with a marked eosinophilia while the other case was a transudate due to a constrictive pericarditis. Francisella tularensis is the causative agent of tularemia. Pleural fluid from three tularemia patients showed a lymphocyte predominant exudate. Bacillusanthracis is the causative agent of anthrax. Cases of inhalational anthrax from a recent bioterrorist attack evidenced the presence of a serosanguineous exudative pleural effusion. These four bacterial microorganisms should be suspected in patients presenting with a clinical history, exposure to known risk factors and an unexplained pleural effusion.
INTRODUCTION
Recent outbreaks in Europe and North America of certain diseases previously thought to be rare or under control have raised concern how prepared the medical community is to identify these diseases. In this review, we describe four unusual bacterial diseases that physicians may encounter: 1) Rocky Mountain and Mediterranean spotted fevers (rickettsia); 2) Q fever; 3) tularemia, and 4) anthrax. The epidemiology, microbiology, clinical characteristics, diagnosis, therapy, and prognosis are described. Based on the review of the literature, we highlight the pleural manifestations and findings that can result from these four diseases.
RICKETTSIAL DISEASES
Rickettsia are a group of small, pleomorphic, Gram-negative coccobacilli belonging to the family Rickettsiaceae of which Rickettsia rickettsiiis the most commonly known. R. rickettsii is the causative agent of Rocky Mountain spotted fever (RMSF) [1]. Mediterranean spotted fever (MSF) is another tickborne, rickettsial disease caused by R. conorii [2]. A single case of pleural disease has been described with MSF in Greece, but none due to RMSF thus far [3]. R. rickettsii could potentially cause pleural disease and, therefore, we include RMSF in this review.
Epidemiology
MSF was first described by Conor and Bruch in northern Africa in 1910 [2]. RMSF was first described among settlers in Montana in 1873 and was the causative organism identified from 1906 to 1910 by Chowning, Wilson and Ricketts [1, 4, 5]. MSF is endemic in the Mediterranean area (Fig. 1). RMSF is endemic in North America (Fig. 1). The majority of cases within the U.S. are concentrated in the south central states of Arkansas, Oklahoma and Tennessee and in the south atlantic region including Maryland, Virginia, North, and South Carolina [1, 5]. Most cases of MSF and RMSF occur during the months of April and September [1, 2, 5]. Both are more prevalent among men and affect both children and adults [1, 2, 4].
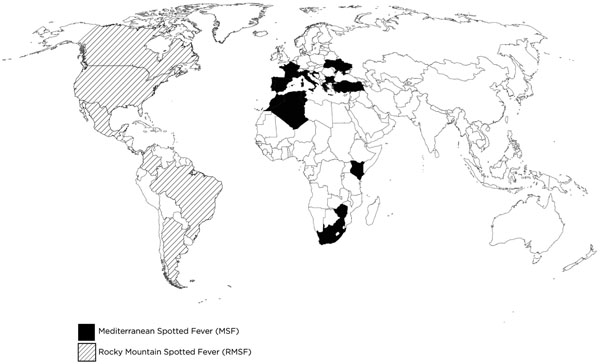
Mediterranean and Rocky Mountain Spotted Fever geographic distribution.
Microbiology
Rhipicephalus sanguineus (brown dog tick) is the main vector for R. conorii transmission [2]. Dermacentor variabilis (American dog tick), Dermacentor andersoni (Rocky Mountain wood tick), and R. sanguineus are the known vectors for R. rickettsii transmission in the U.S. [1, 4, 5]. Ticks are natural reservoirs harboring rickettsia. Humans are accidental hosts. Infection results from the bite of an infected tick. They are obligate intracellular bacteria that localize and multiply in endothelial cells of small to medium-size blood vessels, inducing a lymphohystiocytic vasculitis response [2, 5].
Clinical Presentation
MSF is characterized by the triad of fever, rash, and eschar [2]. RMSF is characterized by the classic triad of fever, headache, and rash [1]. There is an asymptomatic incubation period ranging from two to 14 days for both diseases. Early in the disease, nonspecific symptoms, such as fever, malaise, aching, myalgias, chills, and headache occur. A black eschar at the tick bite site can be present in up to 60% of MSF cases. A rash appears two to five days following fever in both diseases. It is red-purple, macular, and blanching at first, but then becomes maculopapular and involving the palms and soles. The rash spreads centripetally from the wrists and ankles to the arms, legs, and trunk [1, 2].
Diagnosis
The diagnosis is often clinical. For MSF, an eschar is present in about 60% and a rash about 96% of the cases [2]. For RMSF, a rash is present in about 80% and central nervous system manifestations like headache or confusion in about 30% of the cases [4]. The RMSF triad of fever, headache and rash has a sensitivity of 5% within the first 3 days of illness which increases to 60% by the second week [1, 4, 5]. Common laboratory abnormalities include hyponatremia in up to 20% of patients, and almost a third present with anemia and thrombocytopenia [4, 5]. Serology using indirect fluorescent antibody (IFA) is most widely available, but cannot distinguish between different rickettsia. An IgM or IgG IFA titer of 1:64 or greater is considered positive [4]. Its sensitivity increases to about 94% if there is a fourfold or greater rise in convalescent serum IgG or IgM titers obtained between 14 to 21 days [5]. Polymerase chain reaction (PCR) from skin biopsies of eschars has been used in diagnosing MSF [2]. In RMSF, PCR is available in very few laboratories due to high cost [1]. Testing with PCR for MSF and RMSF has not been standardized. Rapid detection and species identification seem likely advantages with PCR use, but their diagnostic sensitivity and specificity varies among individual assays and has not been established [6]. Due to their obligate intracellular nature, MSF and RMSF are difficult to culture routinely and if done, require cell culture techniques that are labor-intensive and under bio-safety level three conditions [4].
Prognosis
Untreated RMSF carries a mortality rate of about 20% (Table 1). Mortality is reduced to 5% after therapy [5]. Severe cases of MSF and RMSF usually develop neurologic manifestations and multi-organ failure [1, 2].
Treatment of Unusual Infections in the Pleura
| Organism | First Choice (Duration, d) | Alternative | Comments |
|---|---|---|---|
| R. rickettsii (RMSF) and R. conorii (MSF) | Doxycyline (5-7) [1, 2, 4, 5] | Chloramphenicol | May be given until 3 days after defervescence |
| C. burnetii (Q fever) |
Acute infection Doxycycline (10-14) [7, 8] Chronic infection Doxycyline and hydroxychloroquine (18 months) [8] |
Trimethoprim/sulfamethoxazole | Clinical cure in chronic infection represents a drop in IgG or IgA phase I antibody titers to 1:200 or less [82] |
| F.tularensis (tularemia) | Streptomycin (7-10) [19, 22, 23] | Doxycycline, ciprofloxacin | |
| B. anthracis (anthrax) | Ciprofloxacin (60) [27] | Doxycycline Doxycycline plus clindamycin, penicillin or rifampin |
Doxycycline alone for cutaneous anthrax; in combination for inhalational, gastrointestinal or soft tissue infection |
Clinical Characteristics and Pleural Fluid Analysis in Unusual Infections of the Pleura
| Organisms | R. conorii[3] | C. burnetii [11] | C. burnetii [12] | F. tularensis[20] | F. tularensis[21] | F. tularensis[21] | B. anthracis[35] | B. anthracis[26] |
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Age (years) | 30 | 41 | 45 | 37 | 66 | 52 | 61 | 94 |
|
|
||||||||
| Gender | Male | Male | Male | Female | Male | Male | Female | Female |
|
|
||||||||
| Location | Greece | Northern Ireland | Spain | United States | Finland | Finland | United States | United States |
|
|
||||||||
| Occupation | Farmer | Bricklayer | Pharmaceuti-cal factory worker; spent weekends in the countryside | Landscaping | Gardener | Retired farmer | Central supply room hospital worker5 | N/A |
|
|
||||||||
| Medical history | Healthy | Healthy | Healthy | Healthy | Healthy | Tuberculosis 20 years ago | Hypertension | COPD, hypertension, chronic renal disease |
|
|
||||||||
| Clinical presentation | Headache, fever, chills, myalgia, dry cough, left pleuritic chest pain | Cough, left sided pleuritic chest pain, anorexia, weight loss | Acute fever, headaches, cough, asthenia, myalgia, arthralgia, weight loss, pleuritic pain | Fever, chills, pleuritic chest pain, myalgias, headache, night sweats, dry cough | Shortness of breath, chest pain, low-grade fever, abdominal pain | Fever, dyspnea | Weakness, chest heaviness, dyspnea, malaise, cough, chills | Fever, fatigue, myalgias, cough, shortness of breath |
|
|
||||||||
| Characterization of pleural effusion$$$ | Exudate | Exudate | Transudate | Exudate | Exudate | Exudate | Exudate | Exudate |
|
|
||||||||
| Radiographic manifestations | Left-sided pleural effusion | Left-sided pleural effusion | Bilateral pleural effusions | Left-sided pleural effusion with ipsilateral hilar adenopa-thy | Right- sided pleural effusion | Right lower lobe infiltrate and ipsilateral pleural effusion | Widened mediastinum, arge bilateral effusions, soft tissue edema | Widened mediastinum, bilateral effusions |
|
|
||||||||
| Pleural fluid analysis | ||||||||
| Color | N/A | Straw | Sanguineous | Sanguineous | Serosanguineous | Serosanguineous | Serosanguineous | |
| pH | 7.1 | 7.45 | N/A | N/A | N/A | N/A | 7.12 | |
| TP (g/dL) | 5.6 | 5.5 | 5.2 | 5.0 | 4.7 | 4.2 | 3.4 | |
| LDH (U/L) | 560 | N/A | 1080 | 573 | 584 | 1264 | 611 | |
| Glucose (mg/dL) | 54 | N/A | 55 | 88.2 | 92 | 147 | 259.2 | |
| Total nucleated cell count (per µL) | 1200 | 2,500 | 952 | 1,850 | 3000 | N/A | ||
| Neutrophils (%) | 73 | 49 | N/A | N/A | N/A | N/A | ||
| Lymph. (%) | 27 | 48 | 90.5 | 71 | N/A | N/A | ||
| Eosinophils (%) | 70 | |||||||
|
|
||||||||
| Serum antibodies | 1:12481 | 1:1,2803 | 1:6404 | 1:6404 | ||||
| IgM | 1:512 | 1:1922 | ||||||
| IgG | 1:1024 | 1:2048 | ||||||
|
|
||||||||
| Pleural fluid culture | N/A | N/A | N/A | F. tularensis | F. tularensis | N/A | B. anthracis6 | B. anthracis6 |
N/A: not available; Lymph.: lymphocytes; IFA: indirect immunofluorescent antibody; TP: Total protein; LDH: Lactate dehydrogenase; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; Alk. Phos: Alkaline phosphatase; IFA: indirect immunofluorescent antibody.
1. Antibodies against phase II form, did not specify whether IgM or IgG.
2. Antibodies were against phase II form, consistent with acute Q fever infection.
3. Initial titer was negative.
4. Initial titer was 1:160.
5. Her space at work was shared with a mail sorting room.
6. B. anthracis identified by PCR.
$$$ According to Light’s criteria and available information.
R. conorii Case with Pleural Disease
Table 2shows the clinical characteristics and pleural fluid analysis findings of the only case of pleural effusion reported in the literature to date due to R. conorii infection. Diagnosis was established by IFA antibody detection on day 28 (see Table 2). The pleural fluid was a neutrophil exudate. The patient was treated with doxycycline and showed significant improvement after the third day of therapy. Radiographic resolution of his pleural effusion was also noted during the following days [3].
Q Fever
Coxiella burnetii is a small, gram-negative intracellular bacteria and the causative agent of Q fever. It is a zoonotic febrile illness with acute and chronic presentations [7, 8]. Q fever is known to cause atypical pneumonia [9]. In 2007, there was an epidemic in the Netherlands, that was associated with goat farming [10]. No case of pleural disease associated with this epidemic has been reported. A patient with eosinophilic pleural effusion was described [11] and another with constrictive pericarditis and bilateral effusions [12]. A case series of Q fever pneumonia in Japan has been reported with one patient presenting with large bilateral effusions [13].
Epidemiology
Almost simultaneously around 1935, two investigators, one in Australia and another in the United States, described a new microorganism that was initially named Q fever for query by the Australian pathologist, Dr. E.H. Derrick. He and McFarlane Burnet investigated a febrile illness outbreak among abbatoir workers, while Davis and Cox were performing a tick transmission study examining RMSF among guinea pigs in the U.S. The new organism was named C. burnetii [7, 14]. It has a worldwide distribution, except for New Zealand and Antarctica where no cases have been reported, and its main reservoirs include cattle, sheep, and goats. Domesticated animals, such as cats, are known reservoirs as well. During pregnancy of these animals, it reaches high concentrations in the placenta; at parturition, it is aerosolized into the environment. It is also excreted in urine, feces, and milk. Inhalation of infected droplets causes Q fever in humans [7, 8]. During the recent epidemic in the Netherlands, there have been over 4,000 cases reported from 2007 until 2010 [10].
Microbiology
C. burnetii targets macrophages and monocytes particularly in the lungs and liver. It is able to survive within the phagolysosome and is also capable of forming spores. It has phase I and phase II forms. The phase II form is recognized by the immune system during acute infection. Phase I bacteria are the virulent and highly infectious forms that escape phagocytosis and are more prevalent during chronic infection [7, 8, 14]. Given its high infectivity, it can only be handled under bio-safety level three containment [14].
Clinical Presentation
Q fever can be acute or chronic. The acute form typically presents as a flu-like illness, pneumonia, hepatitis, and less commonly myocarditis or pericarditis [12]. The chronic form can occur months, or even years, after the initial infection and in 1 to 2% of patients after acute Q fever [10]. Infective endocarditis is the most common presentation of chronic infection. The incubation period is approximately 14 to 40 days. A flu-like illness is the most common form of acute presentation and is characterized by the sudden onset of high fever, chills, rigors, myalgias, headache, and fatigue. Rash is rare, but can be present in as many as 20% of cases and is transient [8]. Q fever may cause pneumonia presenting with fever, cough, and presence of round opacities with pleural effusions [8, 11-13]. Q fever should be considered if a patient with atypical pneumonia has splenomegaly or relative bradycardia [9, 15]. Chronic infection is defined as lasting more than 6 months and almost two-thirds of the cases are associated with infective endocarditis [8].
Diagnosis
Immunofluorescence assay serology is the most available diagnostic method and detects IgM and IgG antibodies against phase II and phase I antigens, during acute and chronic infection, respectively [8]. A four-fold rise in antibody titer is considered diagnostic [7, 10]. Anti-phase II titers of IgG 1:200 or greater and IgM 1:50 or greater are considered positive [8]. In a recent study in the Netherlands, the anti-phase II IgM IFA sensitivity and specificity was 100% and 95.3%, respectively [16]. PCR detects DNA bacteria in patient samples and could be used especially in patients with negative or ambiguous serology and high suspicion of the disease [8]. In such cases sensitivity for PCR is 98% [17]. The modified Duke criteria consider a single blood culture positive for C. burnetii or IgG antibody titers against phase I antigen of more than 1:800 to be diagnostic of endocarditis [18].
Complications and Prognosis
In general, complications are rare and prognosis is good with therapy (Table 1). Patients with a history of valvular heart disease, who have developed acute infection, should have serologic Q fever testing at least every 3 months in order to detect any occurrence of endocarditis [7].
Q Fever Cases with Pleural Disease
Murphy and Richardson [11] reported a patient with a left-sided pleural effusion after pneumonia. Results of the analysis of the pleural fluid which was an exudate with marked eosinophilia, are shown in Table 2. Phase II antibodies confirmed the diagnosis (see Table 2) and after two weeks of tetracycline his clinical condition improved with resolution of the pleural effusion. Bautista-Hernandez and colleagues reported a patient with constrictive pericarditis and bilateral pleural effusions in a patient that was treated with pericardiectomy. Serum IFA IgG and IgM antibodies were positive (see Table 2) and the pericardial biopsy IFA examination revealed C. burnetii. A right-sided thoracentesis was consistent with a transudative pleural effusion, although no data from the fluid analysis was provided. The effusions were due to the constrictive pericarditis caused by Q fever as confirmed by dense interstitial fibrosis with clusters of Coxiella burnetii found on pathologic examination. An echocardiogram done seven days after surgery did not show any signs of constriction and six months after pericardiectomy the patient was asymptomatic [12]. Okimoto and colleagues [13] described four cases of Q fever pneumonia; one had bilateral large pleural effusions. He recovered after a 16 day course of therapy with minocycline.
TULAREMIA
Francisella tularensis, a gram-negative coccobacilli, is the causative agent of tularemia. The infection was initially described in Japan, and later in 1911, was isolated from ground squirrels in Tulare County, California. Edward Francis, who worked at the U.S. Public Health Service, later described the clinical and epidemiological features and the microorganism was later named F. tularensis in 1947 [19]. Tularemia is known to cause atypical pneumonia as well as pleural effusions [9]. It can present as an isolated pleural effusion, as described in two patients or in association with pulmonary infiltrates [20, 21].
Epidemiology
Tularemia has worldwide distribution within the northern hemisphere, including North America, Europe, and Asia. Most cases in the U.S. are concentrated in the south central region including Arkansas, Oklahoma, Texas, Missouri, and Louisiana [19, 22]. Tularemia occurs between May and September and can be transmitted by any of the following: 1) a bite from an infected arthropod; 2) handling of infected animal carcasses; 3) oral consumption of contaminated water or food; and 4) inhalation of infected droplets [19]. Approximately 250 species of mammals, birds, reptiles, fish, and invertebrates act as hosts, such as ticks, biting flies, rabbits and hares, cats, mice, rodents, squirrels, beavers, and muskrats [22]. Most infections occur in men, probably due to gender differences in occupations at risk. Landscapers, farmers, and laboratory personnel are all at risk of airborne infection [19, 22].
Microbiology
There are four subspecies of F. tularensis, with type A seen mostly in the United States and considered the most virulent. It grows well on glucose-cysteine-blood agar with colonies producing alpha-hemolysis and appearing after two to five days [19, 22, 23]. Its incubation period is three to six days. Initially, F. tularensis proliferates locally, invading the regional lymph nodes. It then spreads lymphohematogenously to multiple organs. It is an intracellular pathogen that invades macrophages, where it survives and replicates. They produce focal necrotic lesions with neutrophilic and macrophage infiltration, and can form granulomas with central necrosis [19, 22, 23].
Clinical Presentation
Tularemia can produce various clinical syndromes, which include: 1) ulceroglandular; 2) glandular; 3) oculoglandular; 4) oropharyngeal; 5) pneumonic; and 6) typhoidal. Ulceroglandular, the most common form, is characterized by an infected skin lesion that erupts after a tick bite or animal contact and produces localized lymphadenopathy. The glandular form is similar, but without the skin lesion. Oculoglandular syndrome occurs after conjunctival inoculation causing painful conjunctivitis with pre-auricular and cervical lymphadenopathy. Oropharyngyeal tularemia is acquired after oral ingestion and produces painful exudative pharyngitis or tonsillitis. Typhoidal tularemia is characterized by high fevers, headache, myalgias, vomiting, diarrhea, and is associated with more severe disease with higher morbidity and mortality. Pneumonic tularemia occurs following inhalation or, more commonly, after hematogenous spread from either ulceroglandular or typhoidal disease. Patients with pneumonia develop high fevers, headache, cough and, radiographically, present with patchy infiltrates, lobar consolidation, hilar lymphadenopathy, and pleural effusions [19, 22].
Diagnosis
F. tularensis is problematic to culture given its need to grow in cysteine-rich media and incubation in a carbon dioxide environment [23, 24]. Serology and PCR can be performed with less difficulty. Enyme-linked immunosorbent assay (ELISA) or agglutination methods are considered diagnostic, if there is a four-fold rise in IgG antibody levels between the acute and convalescent period over two to four weeks or if levels are greater than 1:128 with microagglutination or greater than 1:160 with tube agglutination. Serology will not be positive within 10 days of exposure. PCR is more specific and can be performed on different tissue samples [19, 22, 23].
Complications and Prognosis
If untreated (Table 1), tularemia carries a fatality rate up to 30% [22]. Infection with subspecies type A, which is more prevalent in the U.S., carries a higher risk of mortality than infection with other sub-species. Appropriate therapy leads to complete resolution of the disease [19, 23].
Tularemia with Pleural Disease
In their review, Thomas and Schaffner reported that 20 to 30% of patients with pneumonic tularemia have associated pleural effusions as seen on chest radiography [19]. Pleural effusion can be the sole manifestation of pneumonic tularemia or may be accompanied by hilar adenopathy and infiltrates [20, 21]. In the first patient, Francisella tularensis was cultured incidentally from the pleural fluid collected in standard aerobic blood culture bottles with subsequent confirmation by serology (Table 2). The patient was treated with streptomycin for 14 days and her symptoms including the effusion resolved [20]. The pleural fluid is usually a bloody lymphocyte-predominant exudate (Table 2). In the two patients described by Pettersson, pleural fluid adenosine deaminase (ADA) was elevated. In both cases, serology was positive for tularemia (Table 2). Both patients had resolution of symptoms and their effusions after two weeks of intramuscular streptomycin [21]. The fact that in some cases, pleural effusion due to Tularemia can show a high concentration of ADA in the pleural effusion is relevant since this aetiology should be included as a false positive for tuberculous pleural effusion diagnosis.
ANTHRAX
Bacillus anthracis is a gram-positive, rod-shaped bacteria and the causative agent of anthrax. Descriptions of the disease causing illness in herbivore mammals, has been recognized for a long time. It was initially described in Germany and France around 1850; and in 1876, Robert Koch identified the spore forming nature of the bacteria as well as the pathogen/host relationship. Anthrax has been developed as a biological weapon since 1945. In 1979, a fatal accident in a military base in Russia killed several hundred people and livestock [25]. In 2001, a bioterrorist attack killed five of 11 people using letters sent through the U.S. postal mail containing anthrax spores [26].
Epidemiology
Anthrax has worldwide distribution and is usually confined to agricultural regions in developing countries. It has re-emerged more recently in the U.S. following the 2001 bioterrorist attack and in Europe between 2009 and 2010 among injection drug users. Spores in the environment are ingested by herbivores from the soil and grass. Within the host they germinate into the virulent form that replicates and kills the host. Infection occurs following inhalation, ingestion, or contact with spores through skin wounds [27].
Microbiology
B. anthracis spores can remain viable in the soil and environment for decades. Germination of spores into the vegetative form occurs inside macrophages. The virulence of the bacteria is due to its capsule and three particles toxin: a protective antigen, a lethal factor, and an edema factor. The capsule protects against phagocytosis. The protective antigen binds the host cell and later combines with the edema or lethal factor to produce edema or lethal toxins respectively. Edema toxin and lethal toxins undergo endocytosis into the host cell, where they are released and disrupt signal mechanisms resulting in apoptosis [25, 27].
Clinical Presentation
There are four forms of anthrax: 1) cutaneous; 2) gastrointestinal; 3) inhalational and most recently; 4) a soft tissue infection. Cutaneous anthrax is the most common presentation worldwide and frequently resolves spontaneously. An initial painless or pruritic papule appears with exaggerated surrounding edema, which later progresses to a vesicle. Rupture of the vesicle occurs and forms an ulcer covered by a black eschar that sloughs two to three weeks later. Gastrointestinal anthrax results following ingestion of contaminated meat. Spores germinate, causing ulcers in the oropharyngeal region, as well as the gastrointestinal tract. Mesenteric lymphadenopathy and ascites cause obstruction with subsequent bleeding and perforation. Symptoms are non-specific and include nausea, vomiting, diarrhea, and abdominal pain. Inhalational anthrax was classically described in millworkers handling animal hides contaminated with spores. Bioterrorist use is now the greatest risk. Spores are phagocytosed by macrophages within the alveoli that carry them to mediastinal lymph nodes causing hemorrhagic mediastinitis. The incubation period is about four days. Patients present with flu-like symptoms, such as fever, cough, and myalgias which last for another four days. Subsequently, there is dyspnea and hypotension that may lead to death within 24 hours. Chest radiography shows a widened mediastinum or bilateral pleural effusions. Patients can have an elevated hematocrit due to hemoconcentration. Soft tissue infection has been described among subcutaneous or intramuscular heroin users [28]. Spores germinate at the inoculation site and do not produce the papule, vesicle, ulcer or eschar characteristically seen with cutaneous anthrax [27].
Diagnosis
Diagnosis of anthrax can be established using multiple techniques. A confirmed case is identified with compatible clinical findings and isolation of B. anthracis, or having two positive tests using supportive serologic or other methods [27]. The Centers for Disease Control and Prevention ELISA for IgG antibodies against protective antigen in serum has a sensitivity of 97.8% and specificity of 97.6% for the detection of inhalational and cutaneous anthrax [29]. Rapidly growing aerobic and large gram positive bacilli are identified in standard culture media. They form non-hemolytic colonies in sheep’s blood agar, are non-motile, and show susceptibility to penicillin [30]. The specificity of blood cultures is 100%, but cultures from skin or other lesions 60-65% [31].
Complications and Prognosis
If untreated (Table 1), anthrax is a highly fatal disease. Mortality for cutaneous anthrax is reported to be between 5 and 20% (less than 1% if appropriately treated); gastrointestinal anthrax between 25 and 60%; and inhalational anthrax 46% [27, 30]. Shock is a common complication with inhalational anthrax and can occur in almost half the patients [27]. The challenge remains for early identification and institution of appropriate treatment.
Inhalational Anthrax and Pleural Effusions
All 11 confirmed cases of inhalational anthrax were shown to have pleural effusions and a widened mediastinum. Only seven of the 11 cases have been described in detail in the literature. In the seven cases, blood cultures grew Bacillus anthracis and one in cerebrospinal fluid [26, 27, 32-35]. The pleural effusions in all seven cases were serosanguineous and exudative, but pleural fluid analysis was only available for two of the cases (Table 2). Anthrax can also be cultured and identified with PCR from pleural fluid [26, 35]. Therapy for all seven included either ciprofloxacin or levofloxacin in combination with other agents such as rifampin, clindamycin, penicillin G and ceftriaxone. Of the seven cases, five died of multi-focal organ hemorrhages including the mediastinum. The pleural effusions persisted until the time of death.
CONCLUSION
Rickettsial disease, tularemia and anthrax can result in an exudative pleural effusion. Pleural effusion in one case of Q fever due to Coxiella burnetii was found to be a transudate of cardiac origin due to invasion of the pericardium; in another case an eosinophilic exudate was reported. Rickettsial-related pleural effusion results in a neutrophilic-predominant exudate, and tularemia-related pleural effusion in lymphocytic-predominant exudate. As seen in all the cases reviewed, these zoonotic infections can affect healthy individuals. In most, a careful history, and physical examination should alert the clinician to the underlying causative agent. Isolation of the organism from pleural fluid has only been described with tularemia and anthrax.
SUMMARY
Rickettsia, Q fever, tularemia and anthrax are unusual pathogens that can produce respiratory illness. These four bacterial microorganisms should be suspected in patients presenting with a clinical history, exposure to known risk factors and an unexplained pleural effusion.
ACKNOWLEDGEMENT
The authors wish to thank Isabel Kummerfeldt for assistance in developing the Fig. (1).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.


