All published articles of this journal are available on ScienceDirect.
Oxidative Stress Markers in COPD Patients Admitted to Pulmonary Rehabilitation
Abstract
Background:
Chronic obstructive pulmonary disease (COPD) is a pathology, which leads to an irreversible and progressive reduction of the airflow, usually caused by smoking, but only present in 25% of smokers. Some mechanisms involved in the onset and progression of the disease are local and systemic factors such as inflammation, exacerbated immune response and the appearance of oxidative stress. For all these reasons, the use of oxidative stress parameters as progression markers or even as a way to monitor the response of any kind of non-pharmacological interventions, like the use of pulmonary rehabilitation (PR), is feasible.
Aims:
The study aims to determine markers of oxidative stress levels in plasma and erythrocytes in patients with COPD through the application of a PR protocol.
Methods:
The study included 25 patients diagnosed with COPD according to the GOLD criteria with a medical indication of PR and attendance at the gym in San José Hospital, Santiago, Chile. Blood samples were obtained before the start of the protocol, in the 10th session, and at the end of the protocol (20th session). These samples were stored for oxidative stress determinations: FRAP (ferric reducing ability of plasma), F2-isoprostanes, reduced (GSH)/oxidized (GSSG) ratio and antioxidant enzyme activity in the erythrocyte. In all stages, associations between events and clinical parameters in patients have been observed. The clinical parameters assessed were the six-minute walking test (6MWT), maximal inspiratory and expiratory pressure, the BODE index and Saint George’s respiratory questionnaire, which includes quality of life.
Results:
The intracellular and extracellular capacity (GSH/GSSG and FRAP) in patients in PR at the 10th session were 53.1 and 34% higher than basal values, respectively. Only the GSH/GSSG ratio was 38.2% lower at the 20th session, related in part with higher plasma and erythrocyte lipid peroxidation at baseline. This could be due to the high concentration of reactive oxygen species in the first sessions, which has been reported in the literature as the acute effect of controlled exercise. Blood lipid peroxidation was 43.34 and 58.34% lower at the 10th and 20th sessions, respectively, demonstrating the improvements in the oxidative parameters with long-term exercise. With respect to oxidative enzyme activity, superoxide dismutase and catalase showed higher values of activity at the 10th and 20th sessions compared to the baseline. In the clinical parameters of the PR, significant changes were found in the BODE index and Saint George’s questionnaire, with these results being associated with a less predictive mortality score and a better understanding of the disease. This may be because the patients achieved longer distances in the 6MWT and better understood the disease at the end of the PR.
Conclusion:
The goal of this study was to contribute to the pathophysiological basis for further research on COPD patients, a disease of high prevalence in Chile. This study could support the basis for non-pharmacological strategies such a PR.
1. INTRODUCTION
The occurrence of oxidative stress (OS) has been linked to the pathophysiology of multiple human diseases and physiological processes such as aging. Among these diseases, one of the most important is Chronic Obstructive Pulmonary Disease (COPD), which is currently a global public health problem and the fourth leading cause of death and is expected to reach third place in 2020 (GOLD). In Chile, it is the third cause of respiratory death among causes of death in men. This disease is characterized by respiratory symptoms and a persistent airflow limitation, which is caused by respiratory or alveolar airway abnormalities, usually the result of significant exposure to particles or harmful gases. It is inflammatory, non-reversible and progressive (GOLD). Its main cause is cigarette smoke, although the inhalation of other types of particles, such as environmental pollution, becomes very important, especially in Chile. Within the pathogenesis of COPD, the development of oxidative stress, pulmonary, muscular and systemic, plays a key role in the progression of the disease.
OS can be defined as an imbalance between an increase in the production of oxidizing agents and/or a decrease in the endogenous antioxidant capacity, which is produced by both the inhaled reactive oxygen species (ROS) of cigarette smoke and those produced endogenously as a product of the systemic inflammatory reaction that characterizes this pathology.
As COPD progresses, many patients in their moderate or advanced stage become oxygen-dependent to supplement the gasometric alterations they present and to prevent exacerbations; however, it has been demonstrated in recent years that short and long- term oxygen concentrations produce an increase in the production of oxidizing agents, which may aggravate the disease even more.
Currently, the measurement of some OS biomarkers, such as total antioxidant capacity, or some parameters of oxidative injury such as plasma lipid peroxidation, have been associated with clinical variables of progression and/or exacerbation of this pathology. Available studies have focused on long-term exposure under hypoxic conditions in healthy subjects. In these individuals, oxygen-poor environmental conditions (such as those found in expeditions at height) are associated with an increase in ROS and OS production in the systemic circulation, at rest and in exercise. The literature on environmental hypoxia suggests that chronic hypoxemia in COPD patients contributes to OS in the systemic circulation under conditions of rest and post-exercise [1].
Recent studies have indicated that COPD is usually associated with significant extrapulmonary abnormalities, called “COPD systemic effects”. These systemic effects could be clinically relevant and could contribute to a better management and disease understanding. Muscle dysfunction, considered one of the major COPD systemic effects, contributes significantly to exercise limitation in these patients [2]. The identification of this disorder plus nutritional support rich in antioxidants added to exercise would improve the quality of life and prognosis of these patients [3]. On the other hand, OS biomarkers have been mainly measured in lungs, little has been described their systemic effect, and taking into consideration that PR produces several benefits without affecting pulmonary function, it remains to be studied whether any of these benefits are related to the modulation of the systemic OS.
In 2006, Rahman proposed that a rational approach to COPD treatment should also include supplementation with antioxidants, not only to neutralize the increased OS and subsequent inflammatory response, but also to identify the source of the oxidants and delay their generation. This could be done by two strategies: 1) augmenting endogenous antioxidant defenses, and 2) boosting non-enzymatic defenses through diet, drugs or exercise [4].
Current studies have focused on finding therapeutic tools to counteract the undesirable effects of the damage produced by ROS, such as pharmacological or non-pharmacological measures or a combination of both, in order to find the most appropriate treatment for the great diversity of patients with this disease.
Pulmonary rehabilitation is a non-pharmacological therapy: its widespread application in chronic COPD patients should be followed by demonstrable improvements in function (health-related quality of life, functional and maximum exercise capacity) attributable to these programs [5]. Rehabilitation serves as an important component of the management of COPD and is beneficial in improving health-related quality of life and exercise capacity. However, studies should focus on identifying which components of pulmonary rehabilitation are essential, its ideal length and location, the degree of supervision and intensity of training required, and how long treatment effects persist. Recent clinical studies have shown that some markers of DNA damage were higher in the group of non-responders to a 3-week PR program and in those patients receiving oxygen therapy [6]. Regarding oxidative stress markers, PR reduced erythrocyte lipid peroxidation at sessions 10 and 20. In contrast, with 8 weeks of PR in stage IV COPD, the group of patients did not improve exercise capacity [7, 8]. Moreover, the mechanistic and molecular effects associated with oxidative stress occurrence are not well characterized.
The aim of this work was to determine markers of oxidative stress levels in plasma and erythrocytes in patients with COPD through the application of a PR protocol.
2. METHODS
2.1. Study Design and Population
From August 2017 to August 2019, a prospective, controlled study was carried out, which included 25 patients admitted to the Pulmonary Rehabilitation protocol at the San José Hospital, Santiago, Chile. The inclusion criteria included: age greater than 50 years, both genders, ex-smokers, with smoking suspended six months or more with a minimum of 10 packages per year, COPD diagnosed and staged according to the GOLD classification (GOLD ≥ II) (FEV-1 50-79%: walking on level ground, and stop every few minutes to catch your breath.); stable COPD or with a time of 4 weeks or more since last exacerbation; a medical indication of PR and attendance at the gym in the San José Hospital; not having undergone a previous PR protocol at Hospital. The exclusion criteria were: exacerbation of COPD up to 4 weeks before the start of protocol, stable angina with a surgical indication or unstable angina, stroke with incapacitating sequelae to perform the physical activity; disabling orthopedic diseases for physical activity; use of ambulatory non-invasive mechanical ventilation; use of systemic corticosteroids up to 8 weeks of before the start of protocol; development of heart failure NYHA III or IV; liver failure CHILD B, C or awaiting transplantation. Coagulopathy (INR > 1.5); renal failure (creatinine > 2.5); consumption of vitamin supplements in pharmacological doses> 1 month: Vit C> 1g / d, Vit E> 400 IU per day; inspiratory muscle training through threshold valve.
2.2. Protocol of Pulmonary Rehabilitation.
The PR program consists of 20 training sessions, with a minimum of twice per week. After the initial assessment of resistance at the beginning of the training program, the load is estimated at 60% of the maximum (in Kp for the cycloergometers and in Km/h on the treadmill). The patient performs physical activity in both systems (treadmill and cycloergometer) with a duration of 30 minutes per system.
Blood pressure, pulse oximetry, heart rate and respiratory rate are evaluated at the beginning, middle and end of the session, or when the session is interrupted by another event. After cool-down exercises, a final assessment with the same tests was done as the assessment on admission to the training program.
The following clinical parameters will be determined: body mass index (BMI), 6-minute walking test (TM6 min), measurement of maximal inspiratory pressure and peak expiratory flow by spirometry (at least 1-year-old), Saint George’s quality of life test, Dyspnea scale (BORG and MRC), Epworth Index, BODE index score.
2.3. Sample Size Determination
Since the total numbers of COPD patients under the PR protocol were only 25, all of them were included in the study.
2.4. Blood Samples
The initial blood sample was taken on the day of the initial assessment (randomization) of the cardiovascular tests (cycloergometer and treadmill). The mid-protocol sample was taken at the beginning of session 10, and the final sample, after 20 full sessions, on the day of reassessment of the tests performed at the beginning of the protocol.
2.5. Oxidative Stress Markers
Once obtained, the blood samples were transferred under refrigeration (-4 °C) and centrifuged at 700 xg to extract the plasma. This was used to determine antioxidant capacity and 8-isoprotanes [9, 10]. The antioxidant capacity determined was assessed by the determination of the ferric-reducing ability of plasma (FRAP) with a detection limit of 10 uM. The inter-assay and intra-assay coefficients of variation (CVs) for FRAP were 3.0% and 1.0%, respectively. Indeed, plasma 8-isoprostane concentration (pmol/mL), recognized as a reliable biomarker of lipid peroxidation in vivo, was determined using an ELISA kit by neutralization reaction by lipid products antibodies (Cayman, Ann Arbor, MI, USA). The intra-assay CVs were 9.5% and 10.7%, respectively. After plasma extraction, the remaining erythrocytes were hemolyzed in a hypertonic medium (distilled water) and stored at -80 °C for the measurement of lipid peroxidation products [11]. Finally, the intracellular redox status and glutathione (GSH) content was determined by measuring the GSH reduced/oxidized (GSSG) ratio in the erythrocytes by fluorometric reaction through the biochemical block of GSH groups [12].
2.6. Data Analyses
Data were expressed as means ± standard deviation (SD). The analysis of the data with normal distribution was performed comparing the averages by means of Student’s unpaired t-test. Parameters that do not normalize were expressed as medians (interquartile range) and compared using the Mann-Whitney U test. The analysis of the averages between sessions was compared by the ANOVA test for repeated measures. Differences were statistically significant with p < 0.05.
2.7. Ethics Approval and Consent to Participate
The trial design was approved by the local ethics committee of the North Metropolitan Health Service. All patients entered the study after signing the informed consent. The research centered on the Declaration of Helsinki and its amendments, while written ethical informed consent was received from all eligible participants
3. RESULTS
3.1. Clinical Characteristics of the Patients
The clinical characteristics of the patients were separated into COPD II (N = 4), III (N= 7), IV (N = 14). 40% of the patients included were women and 60% were men. Comorbidities, drug therapy by the group and the clinical parameters of the PR were tabulated. No significant differences were obtained between groups (Table 1).
3.2. Antioxidant-related Parameters
Patients undergoing PR had 34.02 and 42.04% higher FRAP values in sessions 10 and 20, respectively, compared to the baseline values. (p < 0.05). Between FRAP values at the 10th and 20th sessions, there were no statistical differences. Regarding the intracellular power system, the GSH/GSSG ratio is 53.09%, greater than session 10 compared to the values obtained at the beginning of the study (p < 0.05). With respect to session 20, 38.24% lower values are shown compared to the values obtained in session 10 (p < 0.05).
|
Clinical Parameters |
COPD II (n=4) |
COPD III (n= 7) |
COPD IV (n=14) |
P Value |
|---|---|---|---|---|
| Age | 61 ± 5 | 69 ± 6.7 | 60.3 ± 9.3 | 0.542 |
| Sex (M:F) | 2/2 | 5/2 | 8/6 | 0.666 |
| BMI | 32.8 | 34.3 | 30.1 | 0.358 |
| Comorbidities | ||||
| DM | 1 | 1 | 3 | 0.976 |
| Tuberculosis complications | -- | 1 | 0.542 | |
| 0Home oxygen delivery | 2 | -- | 7 | 0.663 |
| Heart failure | -- | 1 | 1 | 0.957 |
| Drugs | ||||
| Salbutamol | 1 | 5 | 12 | 0.666 |
| Ipratropium | 1 | 4 | 11 | 0.758 |
| Brexotide | 1 | 2 | 8 | 0.459 |
| Budesonide | -- | 2 | 2 | 0.375 |
| Enalapril | -- | 2 | 2 | 0.584 |
| Glibenclamide | 1 | -- | 2 | 0.396 |
| Metformin | 1 | -- | 3 | 0.895 |
| Aspirin | 2 | 2 | 1 | 0.773 |
| Losartan | 2 | 1 | 1 | 0.625 |
| PR Parameters | ||||
| TM6 min (m) | 470 | 376 | 340 | 0.666 |
| PiM (cm de H2O) | 44.5 | 64.8 | 58.3 | 0.758 |
| FEP (Lt/min) | 325 | 198 | 160 | 0.459 |
Data are non-significant differences between groups. Using Mann Whitney Test to compare values between 2 groups. The differences of values between all groups were tested using ANOVA test for repeated measures.
3.2.1. Lipid Peroxidation
The plasma 8-isoprostane values in patients undergoing PR were 45.34 and 58.34% lower in sessions 10 and 20, respectively, than the values obtained in the baseline sample (p < 0.01). There was no statistical difference between the samples from sessions 10 and 20. The MDA values in erythrocytes were 43.86 and 60.53% lower in sessions 10 and 20 than the values obtained in the baseline sample (p < 0.01).


3.3. Intracellular Antioxidant Defenses
Superoxide dismutase activity values increased by 45.55 and 43.29% in sessions 10 and 20, respectively, compared to the baseline sample. There were no statistically significant differences between sessions 10 and 20 (p < 0.05). Catalase activity enzyme values increased by 28.19 and 29.41%, respectively, compared to the baseline sample. There were no statistical differences between sessions 10 and 20 (p < 0.01). The values of the enzymatic activity of glutathione peroxidase in session 10 compared to the baseline increased by 43.01%, and decreased by 37.64% in session 20 compared to the baseline. With respect to session 20 compared to 10, this enzyme decreased its value by 56.4%.
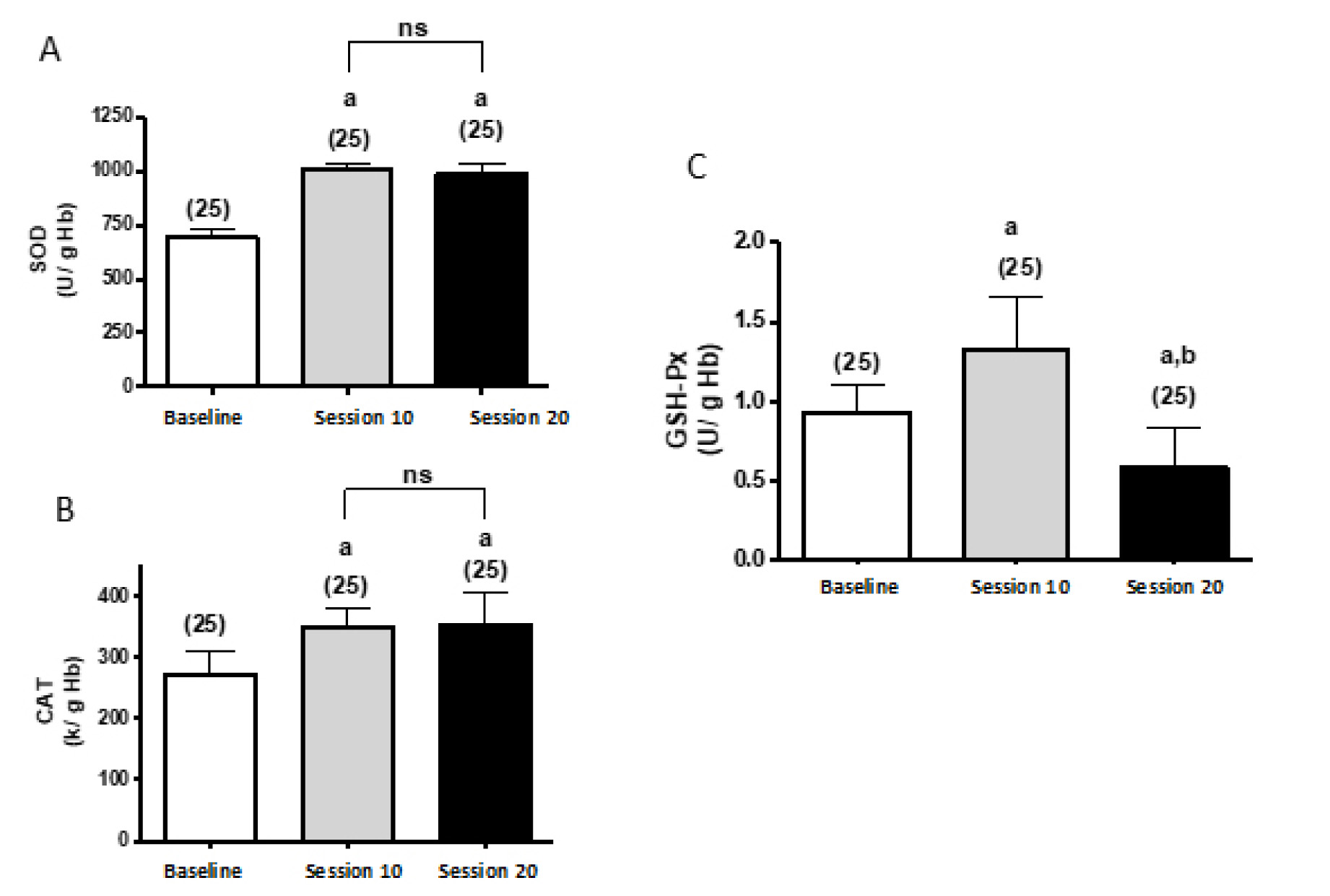
Significant differences: avs basal; bvs session 10
(by two-way ANOVA).
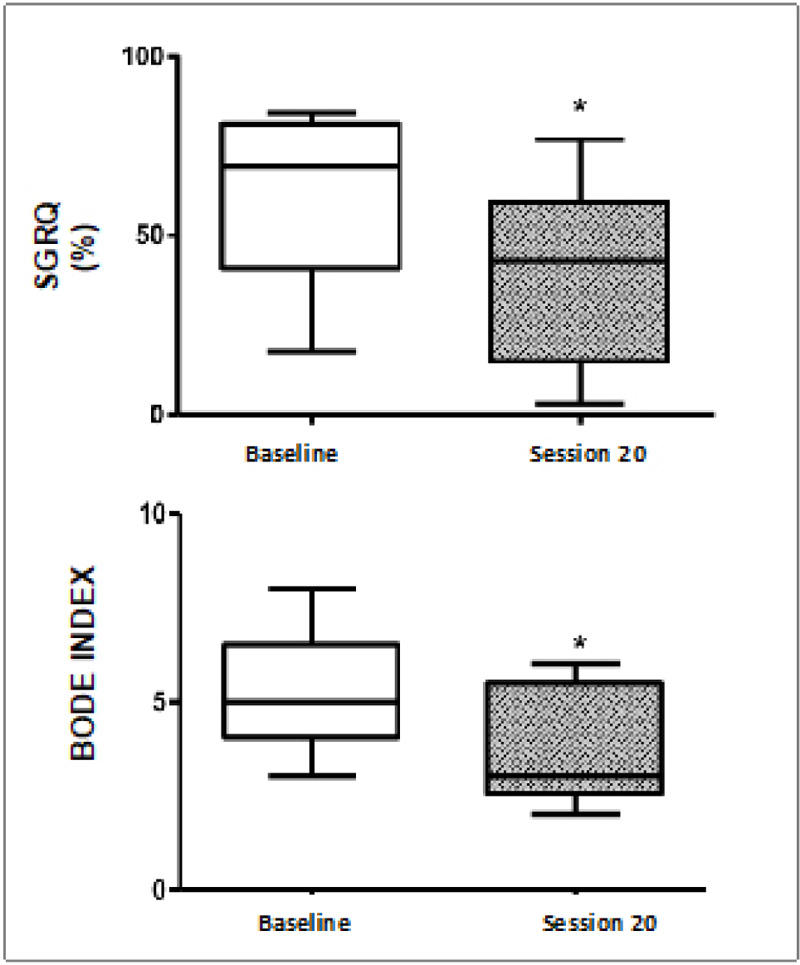
(*p < 0.05. Mann-Whitney U test)
These results indicate lower values of the SGRQ and BODE index at 32.3 and 24.8% compared to session 20 with basal values, respectively (p < 0.05).
4. DISCUSSION
The results presented here show the effect of pulmonary rehabilitation on OS markers in COPD patients. A pulmonary rehabilitation protocol is associated with a lower systemic OS as well as with an enhancement of antioxidant defense systems. Therefore, it is reasonable to state that the stimulation of long-term resistance training would induce an antioxidant response of such magnitude that it could counteract the damage caused by ROS. In this regard, it has been shown that the repetitive exercise stimulus generates a permanent increase in antioxidant activity as well as an improvement in capillary and mitochondrial density, evidenced in active muscle recruitment [13]. In this regard, Radak et al., report that this phenomenon is not paradoxical, but that it is only the result of the adaptation induced by the exercise, involving the activation of the antioxidant system, the blockade of ROS and the repair of biomolecules [14].
Regarding the total antioxidant capacity of the plasma, the results show an increase in FRAP in session 20 (at the end of the protocol) (Fig. 1A). When performing the analysis by degree of severity of COPD by session, both II / III and IV increase, but the latter in smaller magnitude. This could be in part due to the lower antioxidant capacity expressed in more severe COPD. The values of the GSH/GSSG ratio (Fig. 1B) also show this trend; however, its increase is evident only until session 10, a finding consistent with long-term effects on antioxidant mechanisms that would involve a genomic response. This was investigated by Viña et al., who reported long-term increases in oxidized glutathione (GSSG) [15]. In turn, the acute exercise would register an increase in GSSG and plasma MDA values associated with a decrease in GSH [16].
Based on OS biomarkers, plasma lipid peroxidation shows lower values of 8-isoprostanes (Fig. 2A) and MDA (Fig. 2B) in both sessions 10 and 20 compared to the baseline values. Leelarungrayub in 2017 also found lower MDA values in trained subjects after pulmonary rehabilitation and a respiratory training protocol, consistent with the data found in the present study [17]. Although the levels of MDA are higher in COPD patients with exacerbation, if they are compared with stable COPD or with healthy subjects respectively, by decreasing lipid peroxidation products as the PR sessions progress, it could be explained by the decrease in the oxidative load with exercise, reaching stable COPD levels [18].
These findings are consistent with the results shown by Kelsey et al., who also found that in COPD patients after six months of moderate-intensity aerobic exercise, there was a decrease in lipid peroxidation as well as an increase in the total content of reduced glutathione (GSH) [16]. The results of this study show that the GSH/GSSG ratio is higher in session 10 and then decreases to baseline levels in session 20. These values, together with the decrease in lipid peroxidation, could explain that until session 10 there is reduced OS resulting from the combination of the induction of antioxidant enzymes and the GSH pathway. Subsequently, a long-term effect, possibly genomic, predominantly determined by these intracellular enzymes would predominate. GSH, on the other hand, would have a higher consumption; therefore, only the action of these enzymes and other inducible systems would play a fundamental role in the reduction of plasma lipid peroxidation.
In a study conducted with aerobic and resistance exercise, maintained for six months, it was described that the activity of antioxidant enzymes increases steadily compared to baseline levels (pre-training of COPD patient subjects), explaining as a long-term effect, compared to acute exercise, where the enzymatic activity increases after exercise, and quickly falls to baseline levels [19]. Regarding the results of this study (Fig. 3), the activity of the antioxidant enzymes CAT, SOD and GSH-Px is greater after two months of training. As an antioxidant intracellular mechanism, the activity of the GSH-PX is expected to increase, and is thus consistent with that proposed by Paschalis et al. [20].
The strengthening of the antioxidant capacity due to physical training may be because exercise stimulates the expression of genes involved in the regulation of the antioxidant system, mainly Nrf2 (nuclear Factor-E2-related factor 2), a redox-sensitive transcription factor that binds to specific antioxidant response elements (AREs) in the promoter of the gene that encodes for these antioxidant enzymes and in other genes for defense proteins, regulating their expression in many body tissues [21]. It has also been shown that there are significant differences in the antioxidant enzyme content in erythrocytes and in the baseline levels of antioxidant activity in blood between healthy trained and untrained subjects. This suggests that the training status of the subjects influences not only the antioxidant capacity, but also the degree of oxidative molecular damage [22]. Extrapolated to COPD patients, PR would directly affect reducing oxidative damage in these patients since long-term training improves antioxidant defenses and decreases the basal oxidative grade typical of this pathology. Although the baseline levels of oxidants are higher when compared to healthy subjects due to the inflammation maintained in the pathology, among other factors described above. The lipid peroxidation products, such as MDA and 8-isoprostanes, decrease significantly throughout rehabilitation, but in comparison to the increase in antioxidant capacity and antioxidant enzymes as of training session 10, the concept of maintaining antioxidant characteristics as a long-time effect against oxidative injury is reinforced [23].
Another important point, and where more information is described in the face of improvements in PR lies in the topic of quality of life of COPD patients. The main measurement tool is Saint George’s Life Questionnaire, and in the analysis in this study, it is no exception, since a significant improvement was found in all the groups studied, although it was most significant in COPD stage IV, with an average improvement this score. This is consistent with what was described by Nemoto in 2014, where he described that improvements in quality of life do not lead to an increase in OS [7]. It is described that in a PR program, it is expected to obtain a difference equal to or greater than four points expressed by a significant change in the quality of life [24]. Although a score over 50 is associated with higher mortality, in COPD III patients, only 25% obtained a score greater than this value at the beginning of PR, whereas, for COPD IV, 85% of them obtained scores at that cutoff point at the beginning of the protocol. No COPD II exceeded 50 points. This demonstrates that PR significantly improves the quality-of-life aspect measured by the SGRQ questionnaire. And if we add good psychological and nutritional support to this, the most important deterioration aspects of these patients would be seen as a clear improvement with respect to their basal state (Fig. 4).
CONCLUSION
As a projection, it would be a useful and innovative mixed therapy, comprised of a pharmacological intervention with antioxidants and an adjunct therapy with PR protocol to ensure lower long-term oxidative damage or potentiation of antioxidant defenses against nociceptive pro-oxidant injury in these patients.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was approved by the ethical committee of North Heath Services.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was taken from all the participants when they were enrolled.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this research are available from the corresponding author [C.R-.D], upon reasonable request with permission from Ethics Committee.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors are grateful to all respondents who kindly participated in this survey, and to those who directly or indirectly facilitated this research.


