All published articles of this journal are available on ScienceDirect.
Real-life Effectiveness of Omalizumab in Patients with Severe Allergic Asthma: RELIEF Study
Abstract
Introduction:
Patients with severe allergic asthma (SAA) are at risk of severe exacerbations. Omalizumab is recommended as an add-on treatment for patients with uncontrolled SAA, despite high-dose inhaled corticosteroids and long acting β2-agonist combination therapy (standard therapy).
RELIEF was a prospective, open label, multicenter study conducted to assess the real-life effectiveness of omalizumab co-administered with standard therapy in patients with SAA for 24 months.
Methods:
A total of 347 patients aged ≥ 6 years with SAA were enrolled, 285 of whom (8 pediatrics and 277 adolescents and adults) completed this 24-month study. Compared with the 12 months prior to baseline, the mean number of exacerbations was reduced in the overall population at any time interval during the study. Proportion of patients with no exacerbations increased to 77.7% at 24 months from 32.6% at 12 months prior to baseline. A reduction in healthcare resource utilization was also observed. The mean number of specialist visits reduced from baseline (5.8 visits) to 2.4 visits at Month 24.
Results:
The mean asthma control test score was >19 at every time-point during the study. The rate of Global Evaluation of Treatment Effectiveness (GETE) for asthma response significantly increased at Months 18 and 24 (P <0.05) compared to baseline. Pulmonary function remained relatively stable for the overall study population. There were no new or unexpected safety findings in the study.
Conclusion:
RELIEF study showed that add-on therapy with omalizumab is effective in reducing exacerbations, healthcare utilization, and improving GETE score in patients with SAA uncontrolled by standard therapy.
1. INTRODUCTION
Asthma is a common chronic respiratory disorder affecting over 330 million people globally [1]. The GINA 2021 report recommends treatment with a combination of inhaled corticosteroid/long-acting β2-agonist (ICS/LABA) in patients with moderate-to-severe asthma. In addition, add-on therapy with a long-acting muscarinic antagonist (LAMA) is suggested in patients inadequately controlled on high-dose ICS/LABA [2].
Despite current treatment options, some patients with asthma continue to remain symptomatic and experience life-threatening asthma exacerbations [3, 4], highlighting a need for a change in their existing treatment plan. Although only a small proportion (~10%) of the total asthma population is identified as having severe or difficult-to-treat asthma, about 50% of all asthma-related costs are attributed to this population [5].
Omalizumab, an anti-immunoglobulin E (anti-IgE) agent is recommended as an add-on treatment for patients with uncontrolled severe allergic asthma (SAA) (patients aged ≥ 6 years), despite high-dose ICS+LABA combination therapy [6-9]. Previous clinical studies have shown that add-on therapy with omalizumab improves disease control by reducing symptoms and exacerbations [10-12] and is well tolerated in patients with SAA [13-15].
The results of ‘real-world’ studies have complimented the effectiveness and safety data from clinical studies [8, 16]. A 2-year eXpeRience registry showed improvement in the Global Evaluation of Treatment Effectiveness (GETE) score, exacerbation rate, symptoms and rescue medication use in patients with uncontrolled persistent allergic asthma treated with omalizumab [8]. Omalizumab use has been linked with a safe and substantial reduction in the need for oral corticosteroids (OCS) in a long-term follow up study [17]. Recently published real-world studies such as STELLAIR, PROXIMA and PROSPERO, have shown similar benefits of omalizumab [18-20]. Omalizumab is now widely used and has recently reached >1.5 million patient-years of exposure [21].
Even though there is substantial literature available on the effectiveness of omalizumab, the evidence is mostly from Europe and the United States. A more comprehensive understanding is needed on the real-life effectiveness of omalizumab, particularly in South American and Middle Eastern populations, owing to the diversity of these regions. The RELIEF (REal-LIfe Effectiveness) study was conducted to assess the real-life effectiveness of omalizumab co-administered with standard therapy in patients with SAA and to further support the available evidence that confirms the role of omalizumab in improving symptoms in SAA.
2. METHODS
2.1. Study Design and Participants
This was a prospective, open-label, observational multicenter, post-authorization study conducted in patients with SAA treated with omalizumab co-administered with standard therapies (ICS+LABA with or without short-acting beta-agonists [22] and/or OCS, as required). The decision to initiate treatment with omalizumab was independent of this study and physicians prescribed omalizumab in line with the indications and prescribing information available for their respective countries. Patients who were on physician-prescribed omalizumab treatment were enrolled and were prospectively followed up for 24 months. The present study is non-interventional in nature. Subgroups were defined based on age as pediatric group (6 to 11 years) and adolescent and adult group (≥ 12 years); these subgroups were defined for reporting and not for comparison.
The patients were analyzed across 38 centers from 11 countries including Argentina, Brazil, Canada, Colombia, Costa Rica, Kuwait, Mexico, Panama, Peru, Qatar, and United Arab Emirates. The study began in June 2014 (start of data collection) and was completed in May 2018 (end of data collection).
Males and females aged 6-11 years (pediatrics) or those aged ≥ 12 years (adolescents and adults) with physician diagnosed SAA, who had received a minimum of two consecutive doses of omalizumab co-administered with standard treatment, ICS+LABA with or without SABA and/or OCS, as required, were included in the study. By including patients who had received at least two doses of omalizumab, inclusion of patients on chronic treatment was ensured. Detailed inclusion and exclusion criteria are provided in the online supplement.
Patients were followed for 24 months, with 5 recommended visits at baseline (0 month), 4 (flexible visit), 8, 12, 18, and 24 months. The flexible visit at Month 4 was performed only for patients who began the study with less than 16 weeks (4 months) of treatment with omalizumab; as omalizumab demonstrates significant effectiveness at 4 months, this visit helped capture relevant evidence. Patients who had received omalizumab for at least 4 months before enrolment, were not followed-up at the flexible visit, but were otherwise assessed as per schedule for the entire study. Enrolment in the study was considered as baseline and the 24-month follow-up started from enrolment date. All visits and assessments were as per routine care and practice of the treating physician. Patients who discontinued omalizumab treatment for a period of ≥3 consecutive months were considered as withdrawn from the study and were not followed further. This included patients who terminated omalizumab therapy due to lack of effectiveness.
2.2. ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by institutional ethics committees at participating centers and was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines. Written informed consent was obtained from all patients or their legal guardians allowing the use of their data before being included in the study. RELIEF was a prospective non-interventional study, which did not require registration on ClinicalTrials.gov.
2.3. Objectives
The primary objective of the study was to determine the incidence of asthma-related exacerbations (as per the GINA criteria) at 4, 8, 12, 18, and 24 months of omalizumab treatment in terms of mean number of exacerbations per patient per 12 months of follow-up and percentage of patients with zero (or no) exacerbations.
A clinically significant exacerbation was defined as any worsening of asthma considered by the treating physician to require systemic corticosteroids or an increase in the OCS use for patients on maintenance OCS. An exacerbation was considered as severe if there was a reduction in peak expiratory flow (PEF) to <60% of the patient’s predicted or personal best.
Secondary objectives were to evaluate the effect of omalizumab treatment on the clinically significant exacerbations, asthma-related healthcare resource utilization (HRU; number of oral steroid courses, intensive care unit admissions, hospitalizations, emergency room visits, GP visits [independent of the study schedule], specialist visits, days missed from school/work due to asthma, days of work missed by caregivers due to asthma of a child, ambulance services, and intubation), GETE assessment [23], change in asthma control test (ACT) score [24], change in pulmonary function (changes from baseline in forced expiratory volume in one second [FEV1], forced vital capacity [FVC], and peak exploratory flow [PEF]) at baseline, 4, 8, 12, 18, and 24 months and to assess safety (adverse events [AEs] and severe AEs [SAEs]) of omalizumab treatment.
2.4. Statistical Analysis
Since there were no comparison groups and no specific hypotheses were tested, the sample size requirements of the study were based on the precision of the estimate for the primary effectiveness measure. As calculated previously [8], in order to detect required level of effect with 10% precision in the current study and assuming an estimated SD of 4.0, a total of 333 evaluable patients had to be included in the study. Modified intent-to-treat set included all enrolled participants who had ≥ 1 follow-up visit and received ≥ 2 doses of omalizumab. Safety set comprised all patients included in the study who had been exposed to ≥ 1 dose of omalizumab, regardless of the amount of treatment administered. Precision of estimates was assessed with the 95% confidence interval (CI); a 95% CI width±10% of the point estimate was considered to provide an acceptable level of precision.
All analyses were performed using SAS Version 9.4. All continuous variables were summarized by simple descriptive statistics. Number, percentage, and missing categories were presented for categorical data. Statistical tests were applied at 5% significance level. When appropriate, 2-sided 95% CIs for the estimates and the corresponding P-value were provided. McNemar’s test is used to estimate P-value for comparison of the rates of response at a visit to the baseline visit, assuming the rates are dependent.
3. RESULTS
3.1. Patients
In total, 347 patients with SAA were treated with omalizumab and were included in the safety population (12 pediatric patients and 335 adolescent and adult patients) 285 of which completed the study (Fig. 1). The modified intent-to-treat set (mITT) consisted of 301 patients (9 pediatric and 292 adolescents and adults).
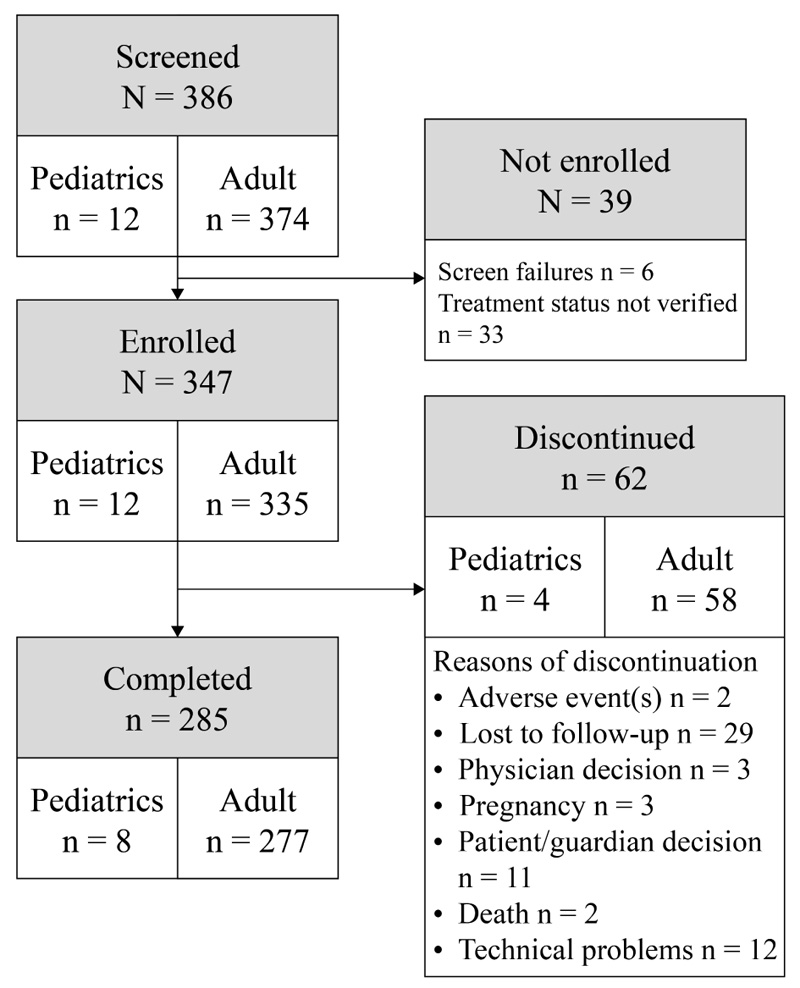
Baseline demographic characteristics are given in (Table 1). The mean age of pediatric patients was 9.4 years and the mean age of adolescent and adult patients was 49.8 years. A high proportion of patients (32.8%; all adolescents and adults) experienced 0 exacerbations in the 12 months prior to the baseline. The mean number of OCS courses (in the 12 months prior to baseline) in the overall population was 4.1±20.42 (pediatric subgroup, 4.4±1.75; adolescent and adult subgroup, 4.0±20.76). The predominant GETE category at baseline was good. The mean baseline ACT scores (19.6±4.82) indicated controlled asthma at baseline in most patients.
The most common allergens (≥ 50% of the overall population) that patients tested positive for at baseline were Dermatophagoides pteronyssinus (144 patients, 70.6%), other allergens (134 patients, 72.4%), and Dermatophagoides farinae (130 patients, 64.4%). At baseline, 29 patients (8.4%) reported prior use of asthma-related medication (pediatric: 1 patient, 8.3%; adolescent and adult: 28 patients, 8.4%).
| Variable | Pediatric (N = 12) | Adolescent and Adult (N = 335) | Overall population (N = 347) |
| Age, median (min-max) | 10.0 (6-11) | 52.0 (12-86) | 51.0 (6-86) |
| Sex, n (%) | |||
| Male | 5 (41.7) | 101 (30.1) | 106 (30.5) |
| Female | 7 (58.3) | 234 (69.9) | 241 (69.5) |
| Race, n (%) | |||
| Caucasian | 10 (83.3) | 240 (71.6) | 250 (72.0) |
| Black | 0 (0.0) | 13 (3.9) | 13 (3.7) |
| Asian | 0 (0.0) | 4 (1.2) | 4 (1.2) |
| Native American | 0 (0.0) | 55 (16.4) | 55 (15.9) |
| Unknown | 0 (0.0) | 1 (0.3) | 1 (0.3) |
| Other | 2 (16.7) | 22 (6.6) | 24 (6.9) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 12 (100) | 197 (58.8) | 209 (60.2) |
| Indian | 0 (0.0) | 1 (0.3) | 1 (0.3) |
| Arabian | 0 (0.0) | 74 (22.1) | 74 (21.3) |
| Lebanese | 0 (0.0) | 2 (0.6) | 2 (0.6) |
| Mixed ethnicity | 0 (0.0) | 3 (0.9) | 3 (0.9) |
| Not reported | 0 (0.0) | 13 (3.9) | 13 (3.7) |
| Other | 0 (0.0) | 36 (10.7) | 36 (10.4) |
| Unknown | 0 (0.0) | 9 (2.7) | 9 (2.6) |
| Body mass index, kg/m2 | 21.7±5.85 | 29.3±6.82 | 29.1±6.93 |
| History of tobacco usage status, n (%) | |||
| Never | 12 (100) | 281 (83.9) | 293 (84.4) |
| Current | 0 (0.0) | 7 (2.1) | 7 (2.0) |
| Former | 0 (0.0) | 47 (14.0) | 47 (13.5) |
| FEV1, L | 2.0±0.46 | 29.2±219.80 | 28±215.23 |
| FVC, L | 2.3±0.62 | 3.7±7.92 | 3.6±7.76 |
| FEV1/FVC, % | 90% | 70% | 70% |
| PEF L/min | 272.7±75.63 | 310.1±116.63 | 308.3±115.17 |
| ACT score | 21.5±4.28 | 19.5±4.83 | 19.6±4.82 |
| GETE category n (%) | |||
| Excellent | 2 (16.7) | 98 (29.3) | 100 (28.8) |
| Good | 1 (8.3) | 154 (46.0) | 155 (44.7) |
| Moderate | 0 (0.0) | 38 (11.3) | 38 (11.0) |
| Poor | 0 (0.0) | 6 (1.8) | 6 (1.7) |
| Worsening | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Absolute eosinophil count/ml | 292±NC | 226.37±150.69 | 228.25±148.87 |
| Serum total IgE, (IU/mL) | 455.9±384.46 | 442.3±627.40 | 442.7±620.37 |
ACT, asthma control test; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; GETE, Global Evaluation of Treatment Effectiveness; IgE, immunoglobulin E; NC, not calculable; PEF, peak exploratory flow.
Pediatrics, aged 6-11 years; adolescents and adults, aged ≥12 years.
3.2. Primary Outcomes
3.2.1. Incidence of Asthma-related Exacerbations
Mean number of exacerbations
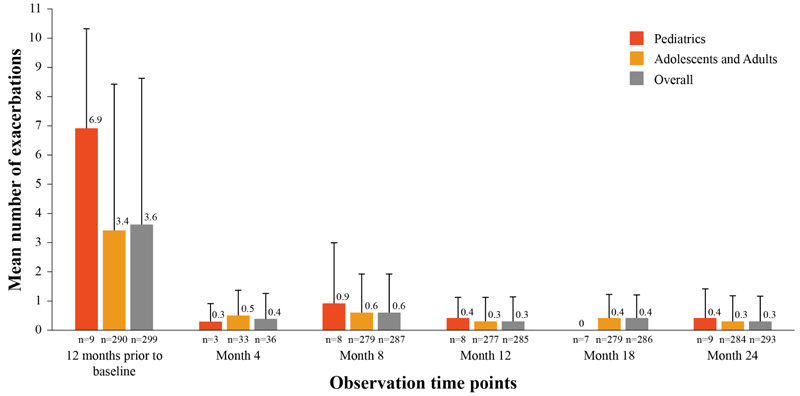
At any given time, the mean number of exacerbations was lower in the overall population and in each subgroup compared with the number of exacerbations reported during the 12 months prior to baseline (Fig. 2).
3.2.2. Percentage of Patients with Zero Exacerbations
In both subgroups and overall population, the percentage of patients with 0 exacerbations at any given time was higher than what was reported at previous observations (Fig. 3).
Visit at Month 4 was a flexible visit and performed only for patients who began the study with less than 16 weeks (4 months) of treatment with omalizumab.

Visit at Month 4 was a flexible visit and performed only for patients who began the study with less than 16 weeks (4 months) of treatment with omalizumab.
3.3. Secondary Outcomes
3.3.1. Proportion of Patients with Clinically Significant Exacerbations
No clinically significant asthma exacerbations were reported in the pediatric subgroup during the study. In the adolescent and adult subgroup, no more than two patients experienced clinically significant exacerbations during any observation interval.
3.3.2. Healthcare Resource Utilization
Asthma related HRU decreased over time compared to baseline for the overall population (Fig. 4). Every healthcare resource had a lower mean utilization over time when compared to 12 months prior to baseline.
The most reported resource at every visit was specialist visits, which at 12 months prior to baseline had a mean overall of 5.8 visits (pediatric: 13.1 visits; adolescent and adult: 5.6 visits), and by Month 24, the overall mean specialist visits decreased to 2.4 visits (pediatric: 3.1 visits; adolescent and adult: 2.4 visits).
In terms of OCS use in the past one year, the mean number of doses in the overall population were 4.1±21.51 oral steroid courses per person at baseline (4.8±1.04 courses, in pediatric subgroup; 4.1±21.81 courses in the adolescent and adult subgroup). Reduction in number of doses was seen as early as four months (0 doses in pediatric subgroup: 2.1±9.35 doses in adolescent and adult subgroup).
3.3.3. GETE Assessment
The GETE score of the pediatric subgroup (n=3) always scored ‘good’ or ‘excellent’ at every visit; however, due to the low number of patients statistical significance in the pediatric subgroup could not be determined. For the adolescent and adult subgroup, the estimated rate of response, defined as a GETE assessment of good or excellent, increased at every time-point (except Month 4). The increase in the rate of response compared to baseline was statistically significant at Month 18 and Month 24 (P <0.05) (Table 2).
3.3.4. Change in ACT Score
The mean ACT score was >19 at every observation time-point for the overall population and in both subgroups. At Baseline, the mean ACT score was 21.5±4.28 for the 8 evaluable patients in the pediatric subgroup, and 19.7±4.80 for the 210 evaluable patients in the adolescent and adult subgroup. The mean ACT score ranged from 23.3±2.22 at Month 8 to 25.0±3.46 at Month 24 in the pediatric subgroup, and from 20.3±4.34 at Month 8 to 20.1± 5.08 at Month 24 in the adolescents and adult subgroup. In the adolescent and adult population, an improvement in the ACT score was statistically significant at Month 8 (P=0.041) compared to baseline. At the remaining time points, the least squares mean (LSM) change was always positive. The change in ACT score was not evaluable for the pediatric subgroup. The analysis of change in ACT score from baseline for overall population is given Table 3.

ER, emergency visits; GP, general practitioner; ICU, intensive care unit Baseline refers to the visit at Month 0
|
Adolescent and Adult N = 292 |
Overall (N = 301) |
|||||
| n |
% (SE) 95% CI |
P value | n |
% (SE) 95% CI |
P value | |
| Baseline | 258 | 0.15 (0.022) 0.11 to 0.20 |
− | 261 | 0.15 (0.022) 0.11 to 0.19 |
− |
| Baseline-Month 4* | 28 | −0.07 (0.07) −0.21 to 0.07 | 1.0000 | 30 | −0.05 (0.07) −0.18 to 0.07 | 1.0000 |
| Baseline-Month 8 | 249 | 0.04 (0.03) −0.01 to 0.10 | 0.1114 | 252 | 0.04 (0.03) −0.01 to 0.10 |
0.1114 |
| Baseline-Month 12 | 244 | 0.04 (0.03) −0.01 to 0.09 | 0.1433 | 247 | 0.04 (0.03) 0.00 to 0.09 |
0.1433 |
| Baseline-Month 18 | 243 | 0.10 (0.03) 0.05 to 0.15 |
0.0007 | 246 | 0.10 (0.02) 0.05 to 0.14 |
0.0007 |
| Baseline-Month 24 | 241 | 0.10 (0.02) 0.05 to 0.14 |
0.0012 | 244 | 0.10 (0.02) 0.05 to 0.14 |
0.0012 |
N, number of patients of intent-to-treat set at each respective group; n, number of patients at each specific time-point of respective group.
Response rate is defined as a global evaluation of treatment effectiveness (GETE) of good or excellent.
*Month 4 is a flexible visit only for patients who began the study with less than 16 weeks (4 months) of treatment
Exact binomial test (Clopper Pearson) is used to estimate 95% CI of the rates of response to treatment for each visit. McNemar’s test is used to estimate p value for comparison of the rates of response at a visit to the baseline visit, assuming the rates are dependent. Difference in rate (SE) and it’s 95% CI will calculated based on proc genmod model.
| - | Month 4* | Month 8 | Month 12 | Month 18 | Month 24 |
| n | 31 | 203 | 200 | 204 | 201 |
| LSM±SE (95% CI for LSM change) | 0.17±0.55 (−0.90 to 1.25) |
0.60±0.28 (0.05 to 1.15) |
0.49±0.31 (−0.11 to 1.10) |
0.45±0.34 (−0.23 to 1.13) |
0.35±0.32 (−0.29, 0.99) |
| P value | 0.7526 | 0.0328 | 0.1096 | 0.1930 | 0.2858 |
CI, confidence interval; LSM, least squares mean; SE, standard error
*Month 4 is a flexible visit only for patients who began the study with less than 16 weeks (4 months) of treatment
| - |
Pediatric (N = 12) |
Adolescent and Adult (N = 335) |
Overall (N = 347) |
| Patients with at least 1 AE | 4 (33.3) | 178 (53.1) | 182 (52.4) |
| Patients with at least 1 SAE | 0 (0.0) | 33 (9.9) | 33 (9.5) |
| Deaths | 0 (0.0) | 2 (0.6) | 2 (0.6) |
| Patients who discontinued due to AEs | 0 (0.0) | 2 (0.6) | 2 (0.6) |
| Patients who discontinued due to SAEs | 0 (0.0) | 2 (0.6) | 2 (0.6) |
| AEs requiring study drug interruption/dose adjustment | 0 (0.0) | 10 (3.0) | 10 (2.9) |
N = number of patients in the safety set; AE, adverse events; SAE, severe AE.
AEs/SAEs occurring on or after study Day 1 and up to 30 days after the discontinuation of the study drug are reported
3.3.5. Change in Pulmonary Function
The pulmonary function of the overall study population remained relatively stable (Supplementary Table 1). Over time, the pediatric subgroup demonstrated an increase in mean FEV1 compared to baseline. The adolescent and adult subgroup initially showed a decrease in mean FEV1 value compared to baseline; however, at Month 24, a slight increase compared to baseline was reported. Over time, the pediatric subgroup demonstrated an increase in mean FVC compared to baseline. FVC for the adolescent and adult subgroup showed a decrease over time and by Month 24, a mean value of 2.7±0.10 L was reported, a change of −0.13±0.71 L compared to baseline. An increase in mean PEF was observed in both subgroups over time compared to baseline.
3.3.6. Safety
There were no new or unexpected safety findings in the study. A total of 52.4% patients experienced at least one AE. A total of 9.5% participants (33 patients; all being adolescents and adults) experienced a SAE, due to which 2 patients discontinued the study (Table 4). The most common AEs by preferred term were asthma (130 patients, 37.5%; all asthma-related AEs, including asthma exacerbations, were grouped under the single preferred term of “asthma”.), nasopharyngitis (11 patients, 3.2%), pneumonia (9 patients, 2.6%), and bronchitis, headache, and upper respiratory tract infection (8 patients, 2.3% each). The most common SAEs by preferred term were asthma (11 patients, 3.2%), pneumonia (3 patients, 0.9%), and bronchitis and gastritis (in 2 patients each).
No patients discontinued due to non-serious AEs. There were 2 reported deaths due to thoracic trauma and liver cirrhosis in the adolescent and adult subgroup. Neither death was related to the study medication.
The RELIEF study provides additional insights on the effectiveness of omalizumab in patients with SAA who remain uncontrolled with standard of care in real-life settings. In this study, the results were categorised into different age groups for reporting purposes and to provide real-world evidence separately for children and adults. The results show that add-on treatment with omalizumab reduces the number of asthma exacerbations and improves HRU over time in pediatric, adolescent and adult SAA patients. Omalizumab also had a positive effect on asthma control and overall improved pulmonary function in patients with SAA.
The results reported in RELIEF study are in line with previously published randomized controlled trials which demonstrated that omalizumab treatment reduces asthma exacerbations and improves asthma control and asthma-related quality of life when added to high-dose ICS and LABAs in patients with severe uncontrolled asthma [11, 14]. In addition, various real-world observational studies from France, Germany, Belgium, UK, and USA have demonstrated similar benefits of omalizumab in real-life clinical settings [9, 16, 25-27].
Generalizability of results from controlled clinical studies to a real-life setting is a concern for almost all therapeutic areas. The RELIEF study was conducted to address the evidence gap between the efficacy outcomes in clinical trials and real-life setting, and to determine how management of a patient population with SAA could be optimized. Most of the real-world studies on omalizumab to-date are country-specific and moreover, there is a paucity in the literature regarding the effectiveness and safety of omalizumab in patients with SAA from Latin American countries and Middle Eastern states, who are the target population of this study.
Asthma exacerbations increase the risk of morbidity and mortality. Allergen-induced exacerbation leads to increased levels of IgE, type 2 (T2) cytokines and infiltration of inflammatory cells [28]. It is clear that IgE is involved during allergic asthma onset and chronic phase of the disease [29]. The addition of omalizumab as an inhibitor of IgE to standard asthma therapy reduces mean asthma exacerbations [30] and is clinically effective in patients with SAA [29]. In the RELIEF study, the mean number of exacerbations during any time interval was notably lower in the overall population and in each subgroup compared to the number of exacerbations reported in 12 months prior to baseline. These results are in agreement with other real-world studies where omalizumab therapy was associated with a significant reduction in asthma exacerbations [12, 31]. In our study, ≤2 adolescent and adult patients reported clinically significant asthma exacerbations during the 24 months of the study. STELLAIR study reported that more than 70% patients showed a ≥40% reduction in annual exacerbation rate after 4-6 months of omalizumab treatment [19]. A substantial reduction in clinically significant asthma exacerbations was observed after 12 and 24 months of omalizumab treatment in the eXpeRience registry [8]. Similarly, a meta-analysis by Holgate et al., demonstrated that omalizumab significantly reduced mean rates of significant episodes of exacerbation per patient-year in high-risk of serious asthma-related morbidity and mortality compared with placebo (P=0.007) [32].
In our study, the mean ACT score was >19 at each time point during the study, indicating well-controlled asthma throughout the study. All patients were prescribed omalizumab before enrolment in the study, and our findings demonstrate that omalizumab is effective in asthma control in the real-world setting. This result is consistent with the findings reported in other real-world studies. In the PROSPERO study, approximately 50% of the ~85% of patients uncontrolled at baseline, reported ACT >19 at the end of the study [20]. The two retrospective studies by Verma et al., and Pilon et al., showed that the number of patients with good asthma control (signified by ACT score >19) were increased in the post- versus pre-omalizumab period [31, 33].
It should be noted that some patients in our study had been undergoing long-term omalizumab therapy prior to the study. These patients would have had more controlled allergic asthma by the time they enrolled in the study. However, it has already been observed in various studies that omalizumab is associated with better asthma control as measured by ACT after omalizumab treatment. In eXpeRience registry, a meaningful improvement in ACT score (increase of ≥3 points vs baseline) was observed from baseline to Months 12 and 24 (change in score 6.1 and 6.2, respectively) after omalizumab treatment [8]. Similarly, in the EXCELS study, for the new users of omalizumab, 61% experienced a clinically important improvement in asthma control during the course of the study, with a majority experiencing that improvement within the first 6 months [34].
Uncontrolled asthma is associated with an increase in HRU that incurs a high socioeconomic burden and direct/indirect costs [35, 36]. The utilization of healthcare can be reduced by employing effective asthma management strategies [37]. Previous randomized trials have reported reductions in hospitalization and emergency room visits after omalizumab treatment [11, 38] and it is encouraging to see the continuation of reduction in HRU and in work/school productivity losses with omalizumab in real-life settings as well [12, 39]. In the current study, after omalizumab treatment, a reduction in healthcare utilization was observed over time as compared to baseline. Similar results were observed in the eXpeRience registry, where asthma-related healthcare utilization (mean±SD, pre-treatment: 6.2±6.97 and Month 24: 0.5±1.28) and school/work absence was reduced during omalizumab treatment [40, 41].
GETE is an accurate predictor of response to omalizumab in patients with SAA [23] and as omalizumab treatment was initiated prior to study, some enrolled patients demonstrated the GETE rate of response (good or excellent) at the time of enrolment. In the RELIEF study, the GETE rate of response (good or excellent), increased at every visit, and became statistically significant from Month 18 onwards. This prolonged time to reach optimal results is a known feature of omalizumab treatment [38]. These findings are consistent with the eXpeRience study, where a majority of patients were reported as responders (excellent/good response) at any time-point during the study [8]. Similar results were reported in STELLAIR study with 67.2% adults and 77.2% minors were reported as responders (i.e. excellent [complete control] or good [marked improvement of asthma]) to omalizumab after 154 days of treatment [19].
The pulmonary function of the overall study population remained relatively stable in this study. This maybe because many patients included in our study were receiving omalizumab for a prolonged period prior to enrolment into the study. However, similar results were also reported by the PROSPERO study, where overall lung function remained relatively unchanged from baseline across the total cohort [20]. This may be because bronchodilator reversibility was not considered as an inclusion criterion for either of these studies which could have resulted in inclusion of patients with low reversibility impacting the effectiveness of omalizumab in improving lung function in these patients. Verma et al. reported that omalizumab treatment results in a significant improvement in FEV1 (0.28 L, P<0.01) compared with one year prior to omalizumab treatment [31]. Overall, our result is in line with the eXpeRience registry, where mean PEF increased by 34.0 L/min at Month 24 from baseline [8]. Among the pediatric patient sub-group in our study, mean PEF increased from baseline by 37.1 L/min at Month 24.
Results of our study confirmed the safety profile of omalizumab with no new safety findings. The most commonly reported AE by preferred term was asthma (37.5%), which was expected for the indication studied. The number of patients with suspected omalizumab-related AEs and SAEs was low. Previous studies have demonstrated the safety profile of omalizumab as an add-on treatment in SAA patients [8, 20].
One of the potential limitations of the study was that this was a single-arm observational study with no comparison arm. However, as the aim of study was to assess the change in effectiveness variables over time during treatment with omalizumab, the use of a single prospective design was appropriate for this study. The number of patients who completed the study was less than the number estimated for the analysis, thus the results of the study should be interpreted accordingly. The status of the patients prior to the onset of treatment with omalizumab was ascertained, and this provided baseline control values that were used to estimate the impact of treatment on the change in asthma severity and outcome measures. As included patients had received omalizumab before we began observation, they potentially tolerated and already responded to treatment. Also, this was an observational study and patients entering already had substantial morbidity, the findings indicate an improvement in symptoms in comparison to the baseline. Moreover, a considerable number of patients had been receiving omalizumab for a long time prior to enrolling in the study, and it is likely that these patients would have had a more stable form of asthma during the study period; hence, results should be interpreted accordingly.
5. INTERPRETATION
Omalizumab has a positive effect on the symptoms and severity of allergic asthma and is effective in reducing asthma exacerbations in a real-life setting.
6. GENERALIZABILITY
Omalizumab has been shown to be effective in controlling SAA in a number of previous studies [8, 11-13, 41]. Our results showed that adding omalizumab to the standard therapeutic regimen in SAA patients reduced the number of asthma exacerbations over time from baseline to the different visits in patients of varying age. Considering the overall safety profile and outcomes from previous studies, the results are regarded as representative.
CONCLUSION
In a real-world setting, omalizumab treatment reduces the number of asthma exacerbations over time in patients with SAA and reduces the utilization of medical resources in pediatric, adolescent and adult patients. Omalizumab also improves asthma control, as determined by ACT score and is safe to use and well-tolerated at the administered doses. These results validate and confirm the role of omalizumab in treating SAA patients who remain uncontrolled on standard of care therapy.
AUTHORS' CONTRIBUTIONS
All authors contributed towards data analysis, drafting, and revising the paper and agree to be accountable for all aspects of the work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by institutional ethics committees at participating. RELIEF was a prospective non-interventional study, which did not require registration on ClinicalTrials.gov.
HUMAN AND ANIMAL RIGHTS
No animals were used that are the basis of this study. All the human procedures were performed in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines.
CONSENT FOR PUBLICATION
Written informed consent was obtained from all patients or their legal guardians allowing the use of their data before being included in the study
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIAL
Not applicable.
FUNDING
The study was funded by Novartis Biociencias S.A.
SUPPORT STATEMENT
The funder of the study contributed to the study design, data interpretation and writing of the report. The corresponding author had full access to all the data and the final responsibility to submit for publication.
CONFLICT OF INTEREST
JKL reports grants and personal fees from Novartis, grants and personal fees from Sanofi, grants from Regeneron, grants from Genentech, grants, and personal fees from GSK, grants, and personal fees from AstraZeneca, grants, and personal fees from Aralez, grants and personal fees from Pediapharm, personal fees from Bausch and Lomb, grants and personal fees from Takeda, personal fees from Merck, personal fees from ALK, outside the submitted work. ACN reports grants from Novartis Brazil, during the conduct of the study. MAA, LMBO, APC, WD, MAV, MJGD, LUG, have no competing interests. NP and PT are employees of Novartis.
ACKNOWLEDGEMENTS
The authors would like to thank the patients, investigators, and staff at participating centers in this study (full list of principal investigators and centers is provided in the online supplementary file). The statistical analysis for the study was conducted by Novartis Healthcare Private Limited, India. The authors thank Pallavi Saraswat, BDS MPH, and Rahul Lad, PhD, for providing medical writing support/editorial support, which was funded by Novartis, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.


