RESEARCH ARTICLE
Self-Reported Sleep Bruxism and Nocturnal Gastroesophageal Reflux Disease in Patients with Obstructive Sleep Apnea: Relationship to Gender and Ethnicity§
Sean Hesselbacher 1, 2, Shyam Subramanian 3, Shweta Rao 4, Lata Casturi 4, Salim Surani*, 5
Article Information
Identifiers and Pagination:
Year: 2014Volume: 8
First Page: 34
Last Page: 40
Publisher ID: TORMJ-8-34
DOI: 10.2174/1874306401408010034
Article History:
Received Date: 6/4/2014Revision Received Date: 5/8/2014
Acceptance Date: 5/8/2014
Electronic publication date: 22/10/2014
Collection year: 2014

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Study Objectives :
Nocturnal bruxism is associated with gastroesophageal reflux disease (GERD), and GERD is strongly associated with obstructive sleep apnea (OSA). Gender and ethnic differences in the prevalence and clinical presentation of these often overlapping sleep disorders have not been well documented. Our aim was to examine the associations between, and the symptoms associated with, nocturnal GERD and sleep bruxism in patients with OSA, and to examine the influence of gender and ethnicity.
Methods :
A retrospective chart review was performed of patients diagnosed with OSA at an academic sleep center. The patients completed a sleep questionnaire prior to undergoing polysomnography. Patients with confirmed OSA were evaluated based on gender and ethnicity. Associations were determined between sleep bruxism and nocturnal GERD, and daytime sleepiness, insomnia, restless legs symptoms, and markers of OSA severity in each group.
Results :
In these patients with OSA, the prevalence of nocturnal GERD (35%) and sleep bruxism (26%) were higher than the general population. Sleep bruxism was more common in Caucasians than in African Americans or Hispanics; there was no gender difference. Nocturnal GERD was similar among all gender and ethnic groups. Bruxism was associated with nocturnal GERD in females, restless legs symptoms in all subjects and in males, sleepiness in African Americans, and insomnia in Hispanics. Nocturnal GERD was associated with sleepiness in males and African Americans, insomnia in females, and restless legs symptoms in females and in Caucasians.
Conclusion :
Patients with OSA commonly have comorbid sleep bruxism and nocturnal GERD, which may require separate treatment. Providers should be aware of differences in clinical presentation among different ethnic and gender groups.
CURRENT KNOWLEDGE/STUDY RATIONALE
Sleep bruxism and nocturnal gastroesophageal reflux disease are common in patients with obstructive sleep apnea, though gender and ethnic differences in the prevalence and clinical presentation have not been well documented. The purpose of this study was to investigate the symptoms associated with nocturnal GERD and bruxism in patients with OSA, and to examine the influence of gender and ethnicity.
STUDY IMPACT
Significant ethnic and gender differences were identified in the prevalence of sleep bruxism and nocturnal GERD in patients with OSA, and in the frequency of associated sleep disorders such as insomnia, daytime sleepiness, and restless legs symptoms. Such differences should be considered when individualizing diagnostic and treatment plans for these patients.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common disease with prevalence ranging between 2-7% in the middle age population with apnea hypopnea index (AHI) > 5, and is even higher among elderly and in patients with cardiovascular complications and metabolic syndrome [1-4]. OSA is characterized by recurrent airway obstruction lasting for longer than 10 seconds during sleep. Whereas, the hypopnea is defined as the drop in peak signal excursion ≥ 30% of the baseline for ≥ 10 seconds in association with ≥ 3% oxygen desaturation or an arousal [5]. Gastroesophageal reflux disease (GERD) is very prevalent among the general population, with approximately 20% of the adults experiencing one or more heartburn symptoms per week [6]. More than 70% of a large survey mentioned nighttime heartburn as a symptom and the majority of them reported sleeping difficulties with heartburn [7].
Bruxism is characterized by stereotypical rhythmic movement of muscles of mastication leading to grinding and clenching of teeth [8]. It is commonly aggravated by stress, sleep disorders, GERD and medications [9, 10]. Sleep bruxism has been associated with arousal response [11] and has been reported to overlap with GERD in many patients [10]. Studies have shown a positive correlation between sleep disordered breathing and tooth grinding [12-14]. There has been mounting evidence of association between GERD and OSA, as well as bruxism and GERD. Gender and ethnic differences in these overlapping conditions have not been looked at. We attempted to examine the associations between, and the symptoms associated with, self-reported GERD and bruxism in patients with OSA, and to examine the influence of gender and ethnicity.
METHODS
A retrospective review was performed after approval by the IRB of the Baylor College of Medicine. Patients referred for evaluation at the Baylor College of Medicine Sleep Center in Houston, Texas between 2008 and 2009 were reviewed. Inclusion criteria were a diagnosis of obstructive sleep apnea (OSA), defined by an apnea-hypopnea index (AHI) > 5 events per hour, and age > 18 years. Records were excluded if the questionnaires were not completed, or if the technical quality or total sleep time during polysomnography (PSG) were inadequate to determine a diagnosis of OSA. Fifty consecutive records in each demographic category (Caucasian males, African American males, Hispanic males, Caucasian females, African American females, and Hispanic females) that met inclusion criteria were selected for review.
Questionnaires and Clinical Assessment
Each patient completed demographic (including self-reported age, gender, and ethnicity) and sleep-specific (including Epworth Sleepiness Scale [15], and self-reported sleep bruxism, nocturnal GERD, insomnia, and restless legs) questionnaires. The patients then had a consultation with a sleep medicine specialist before undergoing an overnight sleep study for clinical suspicion of OSA or other related health consequences.
Polysomnography
Sleep studies were performed using attended comprehensive PSG and positive airway pressure, including recording electroencephalogram, electrooculograms, sub-mentalis electromyogram, airflow, respiratory effort, oxygen
saturation, anterior tibialis electromyogram, and heart rhythm. Recordings were scored by a technologist manually according to the American Academy of Sleep Medicine Scoring Guidelines [16].
Statistics
Comparisons between the means of 2 normally distributed groups were performed with the unpaired t-test. Comparisons between 2 non-normally distributed groups were done with the Mann-Whitney U test. Comparisons among more than 2 non-normally distributed groups were performed with the Kruskall-Wallis test. Two groups of dichotomous variables were compared with the Fisher’s exact test. A P-value of < 0.05 was considered statistically significant.
RESULTS
Demographic and Clinical Characteristics
Demographic data by gender are represented in Table 1. In the overall study group, GERD was reported in 35% and bruxism in 26%. Both males and females in this cohort had significant morbid obesity with a mean BMI of 41 ± 9 in males and 45 ± 9 in females. The average AHI for both males (52.7 ± 38.2) and females (40.9 ± 36.7) fell in the severe category, covering a wide range of disease severity. No differences between genders were seen in symptomatic complaints, including sleepiness (quantified by the ESS), bruxism, GERD, insomnia, or restless legs symptoms. In Table 2, the demographic data are compared by ethnicity. Of note, Hispanic patients had a higher mean AHI (55.5 ± 41.2) and lower prevalence of bruxism (19%) than Caucasians (41.9 ± 36.6 and 35%, respectively). Other objective and subjective data were similar across groups.
Demographics and clinical characteristics by gender.
| Males | Females | P-Value | |
|---|---|---|---|
| Number | 150 | 150 | |
| Age (years), mean ± s.d. | 46.8 ± 10.8 | 51.7 ± 9.5 | < 0.0001 |
| Height (m), mean ± s.d. | 1.75 ± 0.09 | 1.63 ± 0.08 | < 0.0001 |
| Weight (kg), mean ± s.d. | 125.6 ± 32.3 | 119.4 ± 29.6 | n.s. |
| BMI (kg/m2), mean ± s.d. | 41.1 ± 9.4 | 45.1 ± 9.8 | 0.0002 |
| ESS, mean ± s.d. | 13.5 ± 5.8 | 13.5 ± 5.9 | n.s. |
| AHI (events/hour), mean ± s.d. | 52.7 ± 38.2 | 40.9 ± 36.7 | 0.003 |
| SpO2 nadir (%), mean ± s.d. | 71.5 ± 14.0 | 72.3 ± 15.2 | n.s. |
| Bruxism, number (%) | 43 (29%) | 34 (23%) | n.s. |
| GERD, number (%) | 53 (35%) | 52 (35%) | n.s. |
| Insomnia, number (%) | 120 (80%) | 127 (85%) | n.s. |
| RL symptoms, number (%) | 55 (37%) | 53 (35%) | n.s. |
* s.d. = standard deviation; m = meters; kg = kilograms; n.s. = not significant; BMI = body mass index; ESS =Epworth Sleepiness Scale score; AHI = apnea-hypopnea index; SpO2 = oxygen saturation; % = percentage; RL = restless legs.
Demographics and clinical characteristics by ethnicity.
| Caucasian | African American | Hispanic | P-Value | |
|---|---|---|---|---|
| Number | 100 | 100 | 100 | |
| Age (years), mean ± s.d. | 51.2 ± 9.5 | 48.5 ± 11.4 | 48.6 ± 10.4 | n.s. |
| Height (m), mean ± s.d. | 1.70 ± 0.11 | 1.71 ± 0.11 | 1.65 ± 0.08 | < 0.0001* |
| Weight (kg), mean ± s.d. | 128.9 ± 31.9 | 125.7 ± 31.6 | 112.9 ± 27.6 | 0.0005* |
| BMI (kg/m2), mean ± s.d. | 44.7 ± 10.5 | 42.9 ± 9.7 | 41.6 ± 9.0 | n.s. |
| ESS, mean ± s.d. | 13.5 ± 5.1 | 13.8 ± 6.2 | 13.2 ± 6.2 | n.s. |
| AHI (events/hour), mean ± s.d. | 41.9 ± 36.6 | 43.0 ± 34.2 | 55.5 ± 41.2 | 0.028** |
| SpO2 nadir (%), mean ± s.d. | 73.5 ± 13.5 | 72.9 ± 13.8 | 68.5 ± 17.5 | n.s. |
| Bruxism, number (%) | 35 (35%) | 23 (23%) | 19 (19%) | 0.026** |
| GERD, number (%) | 34 (34%) | 40 (40%) | 31 (31%) | n.s. |
| Insomnia, number (%) | 84 (84%) | 82 (82%) | 81 (81%) | n.s. |
| RL symptoms, number (%) | 34 (34%) | 36 (36%) | 38 (38%) | n.s. |
s.d. = standard deviation; m = meters; kg = kilograms; n.s. = not significant; BMI = body mass index; ESS =Epworth Sleepiness Scale score; AHI = apnea-hypopnea index; SpO2 = oxygen saturation; % = percentage; RL = restless legs.
*Significant differences: Caucasian vs Hispanic, African American vs Hispanic.
**Significant difference: Caucasian vs Hispanic.
Correlation of sleep bruxism and nocturnal GERD with other sleep-related factors and complaints vary among ethnic and gender groups and subgroups.
| Sleep bruxism | Nocturnal GERD | ||||
|---|---|---|---|---|---|
| Nocturnal GERD | Insomnia | Restless legs | Insomnia | Restless legs | |
| All | 0.008 | 0.61 | 0.01 | 0.007 | 0.06 |
| Males | 0.19 | 1.00 | 0.009 | 0.29 | 0.38 |
| Females | 0.01 | 0.42 | 0.55 | 0.004 | 0.0499 |
| Caucasian | 0.08 | 1.00 | 0.19 | 0.25 | 0.03 |
| African American | 0.09 | 0.76 | 0.08 | 0.11 | 1.00 |
| Hispanic | 0.28 | 0.047 | 0.19 | 0.17 | 0.18 |
| Subgroups | |||||
| Caucasian males | 0.33 | 0.24 | 0.02 | 0.70 | 0.51 |
| African American males | 0.75 | 0.70 | 0.52 | 1.00 | 1.00 |
| Hispanic males | 0.49 | 0.13 | 0.20 | 0.47 | 0.53 |
| Caucasian females | 0.23 | 0.40 | 0.76 | 0.40 | 0.01 |
| African American females | 0.05 | 1.00 | 0.12 | 0.02 | 0.76 |
| Hispanic females | 0.37 | 0.24 | 0.66 | 0.41 | 0.35 |
Data reported as P-values by Fisher's exact test (bold indicates P < 0.05).
Association of Sleep Bruxism and Nocturnal GERD with Subjective Sleep Complaints
Self-reported sleep bruxism and nocturnal GERD were associated with other sleep disorders and complaints, as shown in Table 3. Bruxism was associated with nocturnal GERD in all participants (P = 0.008) and females (P = 0.01). Hispanics with bruxism were less likely to report insomnia than those without (P = 0.047). Bruxism and restless legs symptoms were significantly associated in all participants (P = 0.01), males (P = 0.009), and Caucasian males (P = 0.02). All Caucasians, African American males, Hispanic males, Caucasian females, and Hispanic females did not show any significant associations between bruxism and other sleep disorders/complaints.
GERD was significantly associated with insomnia complaints in all patients (P = 0.007), females (P = 0.004), and African American females (P = 0.02). GERD and restless legs symptoms were significantly associated in females (P = 0.0499), Caucasians (P = 0.03), and Caucasian females (P = 0.01).
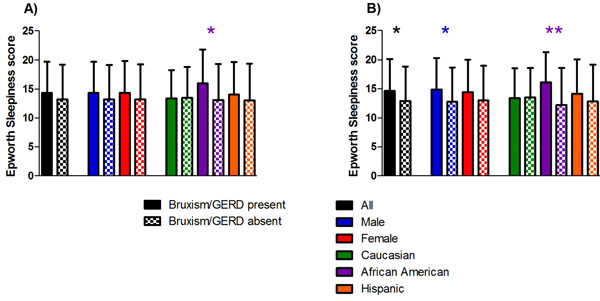
As seen in Fig. (1a), the ESS score was significantly higher in African Americans with bruxism than it was in those without bruxism (16.0 ± 5.8 vs 13.1 ± 6.2, P = 0.036).
This was the only group or subgroup for which bruxism demonstrated a significant association with the ESS. Demonstrated in Fig. (1b), the ESS score was significantly higher in all participants (14.6 ± 5.5 vs 12.9 ± 5.9, P = 0.020), males (14.9 ± 5.4 vs 12.8 ± 5.9, P = 0.040), and African Americans (16.1 ± 5.2 vs 12.2 ± 6.4, P = 0.002) with GERD than those without GERD, with the largest difference being seen in African Americans.
Association of Sleep Bruxism and Nocturnal GERD with Severity of Sleep-Disordered Breathing
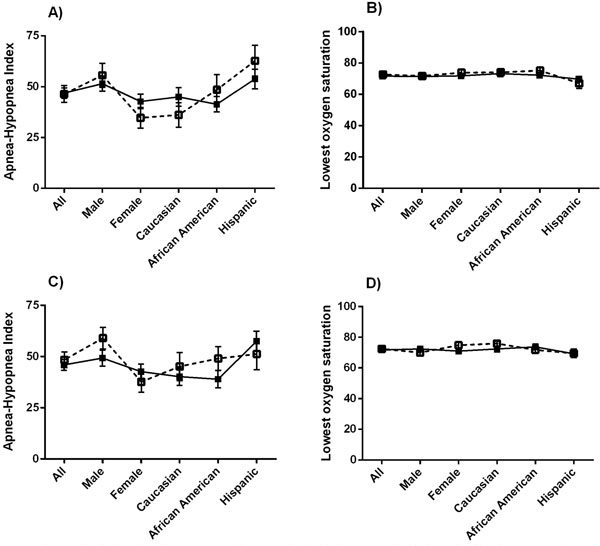
Measures of OSA severity from the overnight PSG were compared with the presence of sleep bruxism and nocturnal GERD. The mean AHI (Fig. 2a) and SpO2 nadir (Fig. 2b) were similar between those with and without sleep bruxism, in all groups and subgroups. Likewise, those that reported nocturnal GERD did not differ in AHI (Fig. 2c) or SpO2 nadir (Fig. 2d) from those that did not report GERD. However, African American males with GERD had a higher AHI (65.8 ± 38.8 vs 45.1 ± 35.0; P = 0.04) and lower SpO2 nadir (68.2% ± 15.2% vs 74.4% ± 13.2%; P = 0.04) than those without, data not shown. No other subgroups demonstrated any significant associations.
DISCUSSION
These data show for the first time some of the differences in symptoms reported by various gender and ethnic groups with sleep apnea in association with sleep-related bruxism and nocturnal GERD. While African Americans reported significantly more daytime sleepiness with bruxism, Hispanics less often reported insomnia. In agreement with prior studies [10,17], patients with bruxism often reported nocturnal GERD. Caucasians with bruxism most often reported GERD, though ethnicity alone did not explain the association with bruxism. Females had the highest association between these two sleep-related symptoms; there was a trend toward an association in African American females specifically. Sleep bruxism was not associated with indicators of OSA severity, similar to previously reported data [14, 18].
Like bruxism, the presence of GERD was not associated with markers of OSA severity, except in African American males. Insomnia was a common complaint in patients with nocturnal GERD; this was especially the case in females and African American females. Sleepiness was also more common in nocturnal GERD, primarily in males and African Americans. The prevalence of restless legs symptoms was similar in all ethnic groups studied, though the symptoms were correlated with sleep bruxism in Caucasian males and with nocturnal GERD in Caucasian females. Sleep bruxism and nocturnal GERD were both more prevalent in our patients with OSA than in the general population [19, 20].
Both sleep-related bruxism and nocturnal GERD have the potential to disrupt sleep and lead to either daytime sleepiness or insomnia. The factors behind an individual or group response to a given sleep disorder are innumerable and will take much more study to delineate. Reported symptoms of sleep-disordered breathing are known to vary across ethnicities and genders [21-24]. It is only when the ethnic and gender subgroups are looked at more closely that we start to see stronger associations, thus highlighting the potentially important differences in clinical presentation among the groups.
As the practice of sleep medicine moves out of the sleep laboratory, and sometimes out of the sleep center entirely, history-taking and personalization of the treatment regimen become more important. Medications and simple diagnostic criteria allow evaluation and treatment of RLS, OSA, and insomnia in the primary care or subspecialist settings, without the use of polysomnography. Without the knowledge of associated or contributing factors, and without the allotted time to perform a thorough sleep review of symptoms, more subtle findings of nocturnal GERD and sleep-related bruxism may go unnoticed and untreated. Cultural or language barriers that inhibit medical history taking could also influence the likelihood of detecting these problems. Insomnia, GERD, and bruxism all have known short- and long-term health consequences, thus magnifying the importance of appropriate diagnosis and treatment.
Nocturnal GERD is often associated with sleep-related bruxism. Case reports have shown that bruxism, and the associated sleep disturbances, improve with treatment of OSA [13, 25]. Previous data has shown that continuous positive airway pressure reduced objective measures of GERD in subjects with or without OSA [26]. Our study did not investigate the effect of OSA treatment on bruxism or GERD, though this may also vary by ethnicity or gender. OSA is thought to contribute to bruxism through high levels of sympathetic activity or by increasing the frequency of arousals. GERD has long been associated with OSA. It has been postulated that the negative intra-thoracic pressure generated during apneic episodes can predispose someone with OSA to increased reflux [27]. Even mild OSA may result in markedly disrupted sleep or generate negative intra-thoracic pressure, so it might be expected that the severity, as measured by AHI and SpO2 nadir, did not correlate with the bruxism or GERD complaints. Obesity is often a contributor to both OSA and GERD. The mean BMI of the patients studied was in the range of morbidly obese in all ethnicities and genders; additionally, the mean AHI fell into the category of severe OSA. These factors likely reflect local referral patterns and may play a role in the proportion of patients that have related comorbidities, such as nocturnal GERD. No association was seen between BMI and GERD complaint in our cohort (P = 0.36). Among the 16 non-obese patients included our analyses, 6 (37.5%) reported nocturnal GERD and 9 (56.3%) reported sleep bruxism; the prevalence of GERD in this small subgroup was similar to the overall study population, though bruxism was much more common. The association of obesity with sleep-related complaints in patients with OSA is another area of future research using an appropriate study population.
Obstructive sleep apnea presents in a variety of ways, with known ethnic and gender variations. Because our cohort was comprised entirely of patients with OSA, it is unknown if our results are applicable to patients without OSA. Since many patients with non-apnea sleep problems are treated in the primary care setting [28-31], application of our findings to the at-large community will be an important next step; future studies should be directed toward this goal. Furthermore, our data and the work of others, have shown that many sleep disorders occur more commonly in patients with OSA; therefore, there should be a low threshold to evaluate for OSA in patients presenting with these symptoms.
Other limitations to the present study include the methods by which the sleep disorders were detected. In order to encourage participation by patients undergoing PSG, the questionnaires were truncated. Because formal diagnostic criteria were not employed, identification of restless legs symptoms in this case is not equivalent to the presence of restless legs syndrome. Likewise, the severity and frequency of the symptoms (GERD, bruxism, insomnia, and restless legs) were not taken into consideration; the participants were asked to mark a box if the symptoms were present. While the threshold to respond is different for each participant, it is presumed that an affirmative response reflects a minimum symptom severity and frequency to have an impact on the patient in a meaningful way. Detection of bruxism by electroencephalographic artifact, GERD by pH probe, or quantification of periodic limb movements during sleep as a surrogate of RLS would provide objective measures of these comorbidities, but would not assess the clinical impact. Also, bruxism and RLS are diagnosed by clinical criteria; PSG features are neither sufficient nor necessary. A small study of 18 bruxers reported frequent tooth-grinding during sleep, with tooth wear noted in large majority of them; asymptomatic controls did not exhibit these findings. Additionally, those reporting bruxism had significantly more frequent bruxism events recorded during PSG [32]. In contrast, GERD symptoms do not correlate as well with objective data. GERD symptoms, including nocturnal heartburn, were compared with 24-hour pH probe in 336 patients. Nocturnal heartburn was independently associated with abnormal findings on pH probe; however, 51% of patients with severe GERD symptoms did not have findings consistent with pathologic GERD [33]. Our study was performed at a single sleep center, which maintains consistency in scoring and implementation of the tests, but the ethnic groups in the study groups (Caucasians, Hispanics, and African Americans) were limited to the local population. Given the absence of participants from other ethnicities, the conclusions are not necessarily applicable to these other groups.
The number of associated sleep disorders evaluated was purposefully limited in our study; the intention was to focus on clinically meaningful associations and symptoms. However, any number of sleep-related symptoms can potentially be worsened by sleep-related bruxism or nocturnal GERD. Similarly, the presence of other conditions, including medical comorbidities or substance abuse, can be expected to exacerbate these sleep problems. Of note, a recent systematic review did not find a relationship between psychosocial disorders and sleep bruxism [9]. Future research may be geared towards identifying other important associations with these sleep disorders, further advancing our understanding of common mechanisms and defining at-risk populations.
CONCLUSION
Both sleep bruxism and nocturnal GERD are common in patients with OSA. These symptoms may overlap in the same patient or present in conjunction with other sleep disorders, all of which may require treatment. Differences in symptom presentation between ethnic and gender groups can make identification of these sleep disorders more difficult; however, understanding these differences may help providers more effectively treat a variety of patient populations.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.