All published articles of this journal are available on ScienceDirect.
Inhalational Steroids and Iatrogenic Cushing’s Syndrome
Abstract
Bronchial asthma (BA) and Allergic rhinitis (AR) are common clinical problems encountered in day to day practice, where inhalational corticosteroids (ICS) or intranasal steroids (INS) are the mainstay of treatment. Iatrogenic Cushing syndrome (CS) is a well known complication of systemic steroid administration. ICS /INS were earlier thought to be safe, but now more and more number of case reports of Iatrogenic Cushing syndrome have been reported, especially in those who are taking cytochrome P450 (CYP 450) inhibitors. Comparing to the classical clinical features of spontaneous Cushing syndrome, iatrogenic Cushing syndrome is more commonly associated with osteoporosis, increase in intra-ocular pressure, benign intracranial hypertension, aseptic necrosis of femoral head and pancreatitis, where as hypertension, hirsuitisum and menstrual irregularities are less common. Endocrine work up shows low serum cortisol level with evidence of HPA (hypothalamo-pituitary-adrenal) axis suppression. In all patients with features of Cushing syndrome with evidence of adrenal suppression always suspect iatrogenic CS. Since concomitant administration of cytochrome P450 inhibitors in patients on ICS/INS can precipitate iatrogenic CS, avoidance of CYP450 inhibitors, its dose reduction or substitution of ICS are the available options. Along with those, measures to prevent the precipitation of adrenal crisis has to be taken. An update on ICS-/INS- associated iatrogenic CS and its management is presented here.
INTRODUCTION
Bronchial asthma and allergic rhinitis are the common clinical problems seen in patients with naso-bronchial hyper-responsiveness. Inhaled corticosteroids (ICS) and intranasal corticosteroids (INC) are the commonly prescribed medications for these conditions. Role of ICS in the management of chronic obstructive pulmonary disease (COPD) is controversial, though they are commonly prescribed along with inhalational bronchodilators. Even though ICS and INS are considered to be safe, systemic side effects can occur, that includes iatrogenic Cushing syndrome. This side effect, although well known, still remains an under-recognized cause of Cushing syndrome. Cushingoid features with evidence of adrenal suppression almost always indicate iatrogenic Cushing syndrome due to exogenous steroid administration. This review highlights the pathogenic mechanisms, clinical features, diagnostic evaluation and management of iatrogenic Cushing syndrome secondary to use of ICS and INS.
BRONCHIAL ASTHMA AND INHALATIONAL STEROIDS
Bronchial asthma (BA) is characterized by airway edema, mucus hyper secretion, and cellular infiltration, along with bronchospasm [1]. This inflammatory reaction can lead to reversible airway obstruction in patients with BA, andCorticosteroids, the powerful anti-inflammatory agents target this mechanism.
Systemic corticosteroids are used only for severe exacerbations and for chronic maintenance treatment of patients with severe BA, because of the higher incidence of side effects. The invention of inhalational corticosteroids in the 1970s and various convincing clinical trials during the late 1980s demonstrated its superiority over other classes of drugs used in asthma treatment [2]. It showed a reduction in mortality and morbidity in patients with BA. The first Global Initiative for Asthma consensus in the early 1990’s further emphasised the significance of ICS in the treatment of asthma [3].
The major advantage of delivery of steroids by inhalational route is the reduced incidence of systemic side effects and therefore, practically ICS have replaced systemic steroids in the treatment of BA, except during emergencies. The dose of inhaled steroid is much lower than the oral dose (ratio 1:20) required to achieve the same therapeutic effects [4].
Various clinical trials proved that ICS in Bronchial asthma significantly reduce the inflammation and hyper-responsiveness of airways, thereby improving lung function, decreasing the severity of symptoms and occurrence of acute exacerbation [5, 6].
MECHANISM OF ACTION OF STEROIDS IN BA
Corticosteroids interfere with the various pathways involved in the process of airway inflammation in BA, by binding to specific DNA sequences [7]. This binding leads to alteration in gene transcription and protein synthesis, resulting in reduction of airway inflammation, by reducing the production of various inflammatory mediators from cells like macrophages, eosinophils, lymphocytes, mast cells and dendritic cells [8, 9].
ROLE OF ICS IN COPD
Chronic obstructive pulmonary disease (COPD) is characterized by air flow limitation that is not fully reversible, in contrast to bronchial asthma, which is associated with reversible airway obstruction due to airway hyper-responsiveness and inflammation [10, 11]. In bronchial asthma ICS is the mainstay of treatment but in COPD, its role is still controversial [12].
Many patients with COPD share the features of airway hyper-responsiveness with BA, leading to considerable overlap between these two, and this is the basis of Dutch hypothesis which states that COPD and bronchial asthma are part of the spectrum of the same basic disease. But the British hypothesis states that both are entirely different [13, 14].
Role of ICS is controversial in patients with COPD and there is no definite evidence to say that it may alter mortality. But some studies showed that it may delay the rate of decline of lung function and reduce the frequency of exacerbation [15].
ICS should be considered in COPD patients with severe disease, frequent exacerbations despite optimal therapy with bronchodilators and those having significant reversibility of airway obstruction with inhaled bronchodilators (i.e., mixed asthma and COPD group) [16].
Use of ICS are associated with increased risk of pneumonia, tuberculosis, easy bruising, osteoporosis, diabetes, oro-pharyngeal candidiasis, hoarseness of voice and even iatrogenic CS in patients with COPD, where the risk-benefit ratio has to be considered before starting steroids [17-24].
FATE OF ICS IN THE BODY
Use of various delivery systems for inhaled corticosteroids results in high amount of oro-pharyngeal deposition of the drug (up to 80% with pressurized metered-dose inhalers [MDI] and dry powder inhalers) that is subsequently swallowed, absorbed from GIT, undergo first-pass metabolism in the liver, and reaches the systemic circulation leading to systemic side effects [25]. Use of spacer device can reduce the amount swallowed to as little as 10%. Rinsing the mouth after the use of ICS can also reduce the oro-pharyngeal deposition of the drug. The oral bioavailability of older ICS are high, as in beclomethasone, which is about 20%, compared to newer drugs like, fluticasone where it is about 1%, and mometasone where it is less than 1% [26]. Ciclesonide, a newer ICS which is a pro-drug, becomes activated only after getting deposited in the lung tissues. ICS are also directly absorbed from the lung after deposition in the respiratory tract following inhalation [27].
Alveolar deposition of ICS give rise to higher systemic exposure compared to the more proximal deposition in the airways. This is because the particles deposited in the ciliated airways are cleared by muco-ciliary clearance mechanism, and the barrier to diffusion in the ciliated airways may be less permeable that in the alveolar air spaces. Making average diameter of aerosol smaller reduces the amount of oro-pharyngeal deposition of the drug, and increases the alveolar deposition [28, 29]. The total amount of steroids that enters the systemic circulation, is the sum of the quantity that reaches the systemic circulation from the oropharayngal deposition of the ICS, after absorption in the GI tract and the first-pass metabolism in the liver (oral bio–availability), plus the quantity that is absorbed directly from the lungs (pulmonary bio-availability). The fate of ICS in the body is shown in the Fig. (1).
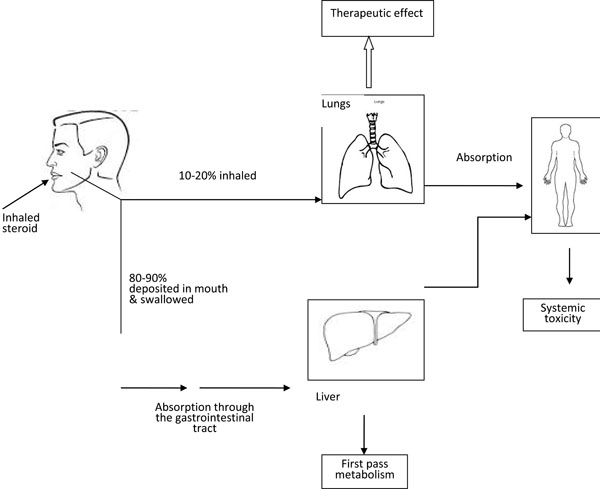
The fate of Inhaled corticosteroids in the body.
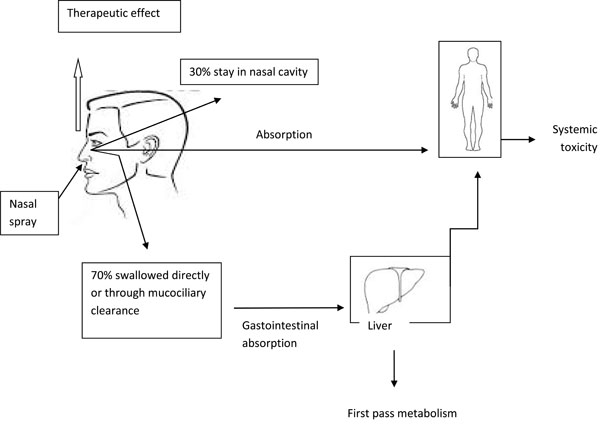
Fate of Intranasal corticosteroids in the body.
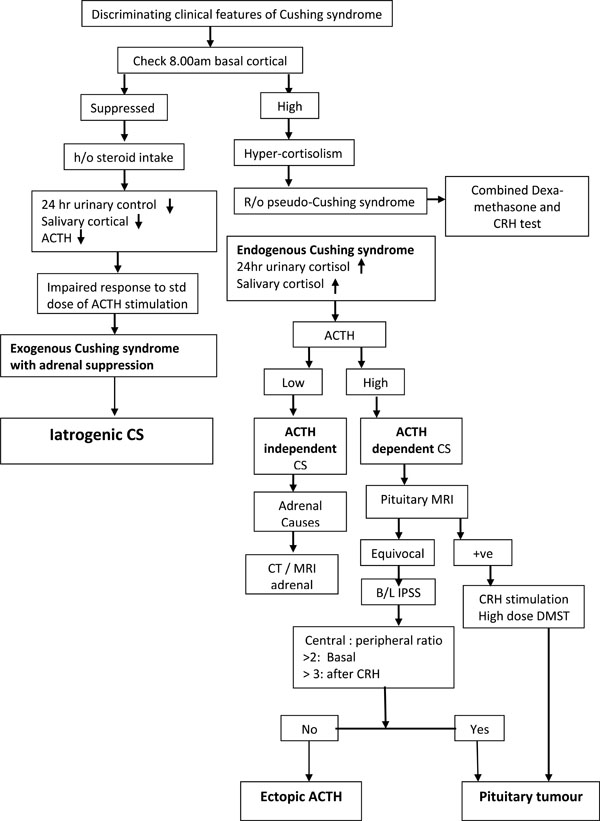
The diagnostic evaluation of CS.
PHARMACOLOGICAL PROPERTIES OF THE ICS
The commonly available ICS are beclomethasone, budesonide, fluticasone, flunisolide, mometasone, triamcinolone, ciclesonide and budesonide [30]. The choice of ICS depends on a number of factors, including potency, systemic absorption, delivery system and the cost. Even though ICS are considered to be relatively safe, various local and systemic side effects are reported [31].
Lung deposition refers to the amount of inhaled drug that get deposited in the lung and act at the site of inflammation. So a high lung deposition of ICS is a desirable property, leading to high therapeutic efficacy. Various factors influence the pulmonary deposition of ICS [32]. They are (1) the physical properties of the ICS; (2) the drug delivery system; (3) particle size and (4) patient characteristics such as age, inhalational technique, and the severity of asthma. Ciclesonide and beclomethasone are having greatest lung deposition [33].
If the particle size of ICS is between 1-5 μm, they are deposited in the bronchi and bronchioles more likely, where as particle size of >5 μm are more likely deposited in the oropharayngal cavity, and very small particles (<1 μm) are either deposited in the upper airways or exhaled out [34]. So the particle size determine both therapeutic efficacy and safety profile of ICS. The smallest particle sizes is for Beclomethasone and ciclesonide delivered by MDI.
Ciclesonide and BDP are pro-drugs which are inactive when inhaled, and subsequently activated into des-ciclesonide and 17-BMP by esterase enzyme present in the lung epithelium. These pro-drugs are inactive till they are activated by esterase enzymes in the lung; and hence associated with lower incidence of side effects compared to other ICS administered in the active form (e.g., fluticasone and budesonide). Within the oro-pharynx the bio-activation of ciclesonide is very low, resulting in fewer incidence of systemic side effects compared to budesonide and fluticasone [35].
The degree of plasma protein binding of ICS reduces their potential for systemic side effects because, the pharmacological activity depends only on the amount of free drug. The protein binding is rapid and reversible, and is generally to albumin. Therefore, high degree of plasma protein binding reduces the systemic side effects. Since Ciclesonide and des-ciclesonide are highly protein-bound (99%) their systemic side-effects and adrenal suppression are minimal [36, 37].
After systemic absorption, ICS are rapidly metabolised by the liver. Maximum clearance rate by the liver of about 90 L/hr is seen, which is equal to the hepatic blood flow. A short-half life and a high metabolic clearance rate reduce the systemic toxicity. The elimination half-lives of the ICS varies from 14.4 hours for fluticasone to as short as 0.5 hours for des-ciclesonide.
The pulmonary residence time (PRT) is the average time required for absorption of the molecules in to the systemic circulation after getting deposited in the lung [38]. PRT depends on lipophilicity and lipid conjugation of ICS. Lipophilicity helps ICS to pass through the cell membrane and, thereby increases the PRT and the volume of distribution of the drug [39]. Higher PRT increases the clinical efficacy of the ICS. However, increase in the volume of distribution after systemic absorption, leads to accumulation of the drug in various tissues causing increased risk for systemic toxic effects.
The lipid conjugation or fatty acid esterification is the process by which ICS form a reversible chemical bond with the fatty acid in the pulmonary tissue [40]. After lipid conjugation the drug-lipid complexes are retained there, making it available for binding to glucocorticoid receptors (GR) for longer duration (similar to a slow release preparation) prolonging the PRT of the ICS, making it convenient for single daily dosing [41]. Therefore, lipid conjugation of the ICS helps to improve the therapeutic efficacy, decreases the risk of development of systemic side effects and prolongs the duration of action.
ALLERGIC RHINITIS AND INTRANASAL CORTICOSTEROIDS (INC)
Intranasal corticosteroids (INC) are the main stay of treatment in patients with Allergic Rhinitis and Chronic Rhino Sinusitis [42, 43]. The INCs are available for treating allergic rhinitis since 1974. The commonly used INC for the management of allergic rhinitis includes: beclomethasone dipropionate, budesonide, ciclesonide, flunisolide, fluticasone furoate, fluticasone propionate, mometasone furoate, and triamcinolone acetonide [44].
MECHANISM OF ACTION OF INC
The clinical efficacy of INC is because of its anti-inflammatory effects [45]. Use of INC reduces the polyp size as well as the nasal symptoms, including nasal congestion, sneezing, rhinorrheoa, loss of smell, and postnasal drip in patients with chronic rhino-sinusitis with nasal polyps [46, 47].
FATE OF INS IN THE BODY
The fate of INC in the body is shown in the Fig. (2).
The systemic absorption rates are highest among the relatively older compounds like flunisolide, beclomethasone, and budesonide, where there is about 33% to 50% absorption of intra-nasally administered dose. The more lipophilic newer compounds like fluticasone propionate, and mometasone furoate, undergo rapid and extensive first-pass metabolism leading to negligible systemic absorption [48].
The order of lipid solubility for corticosteroids is as follows: mometasone furoate > fluticasone propionate > beclomethasone dipropionate > budesonide > triamcinolone acetonide > flunisolide. Lipophilicity of Ciclesonide and des-ciclesonide are greater than fluticasone propionate.
Increased lipophilicity correlates with a greater deposition of the corticosteroid molecule in the nasal mucosa, greater binding affinity for and prolonged occupation of the GR, and, consequently, there is less unbound drug to interact with systemic GR, thereby potentially reducing the risk of systemic adverse effects. The intranasal lipid conjugation or fatty acid esterification increases the local residence time of the steroid molecules, and thereby allows once-daily dosing of many of the INC [49, 50].
METABOLISM OF STEROIDS IN THE BODY AND DRUG INTERACTIONS
The corticosteroids are metabolised in the liver with the help of cytochrome P450 enzyme system, mainly by the iso-enzyme cytochrome P450 3A4 (CYP3A4) [51]. The drugs that inhibit this iso-enzyme increase the serum levels of corticosteroids, when co-administered. Similarly the inducers/stimulators of this iso-enzyme decrease the plasma steroid levels [52]. The drugs that inhibit CYP3A4 are shown in Table 1. The inhibitors of cytochrome P450, when co-administered, can increase the bio-availability of ICS/INS, leading to increased level of steroids in the systemic circulation, resulting in increased risk of systemic adverse effects. Among the inhaled CS, fluticasone and budesonide are mainly metabolized by CYP3A4. Beclomethasone is hydrolysed, and not metabolized by the cytochrome P450, making this as a better choice in patients requiring inhaled therapy, while on a cytochrome P450 inhibitor [53, 54]. The lower risk of flunisolide for drug interaction is because of its weak CYP3A4 metabolism, low glucocorticoid receptor affinity, low lipophilicity and short elimination half-life.
Medications that alters the plasma glucocorticoid levels.
| Medications that Alter the Plasma Glucocorticoid Levels |
|---|
| Inducers of Cytochrome P450: Decreases GC Level |
|
| Inhibitors of Cytochrome P450: Increases GC Level |
| 1. Inhibitors of cytochrome P450 dependent CYP 3A4 inhibitors |
|
| 2. Inhibitors of cytochrome P450 dependent CYP 2D6 |
|
A Comparison of Spontaneous Cushing Syndrome and Iatrogenic Cushings syndrome.
| Clinical Features | Spontaneous Cushing’s Syndrome | Iatrogenic Cushing’s Syndrome |
|---|---|---|
| H/o exogenous steroid intake | Absent | Present |
| Onset and progression | Develop gradually | More abrupt with striking features |
| Psychological features | Less severe | More severe |
| Hypertension | More common | Less common |
| Hirsutism, virilising feature | More common | Less common |
| Menstrural irregularities | More common | Less common |
| Glucoma, ocular features | Less common | More common |
| Avascular necrosis | Less common | More common |
| Benign intracranial hypertension | Less common | More common |
| Pancreatitis | Less common | More common |
| Osteoporosis | Less common | More common |
| Spinal epidural lipomatosis | Uncommon | Rarely reported |
| Serum cortisol | Increased | Suppressed |
| ACTH | Increased / decreased | Suppressed |
| Adrenal suppression | Absent | Present |
Properties of an ideal inhalational standard.
|
Ritonavir, used for the treatment of HIV infection is a potent inhibitor of the hepatic CYP3A4 iso-enzyme. It is usually used at low doses to boost the levels of other protease inhibitors (PIs) in patients with HIV infection. Because of its potent hepatic CYP3A4 iso-enzyme inhibitory property, ritonavir and other PIs can result in various drug–drug interactions [55]. Since corticosteroids, such as ciclesonide, fluticasone, mometasone and triamcinolone, are mainly metabolised by hepatic CYP3A4, their metabolism will be inhibited when co-administered with ritonavir; leading to steroid accumulation, iatrogenic Cushing's syndrome (CS); and adrenal suppression. Co-administration of medications such as itraconazole, verapamil and diltiazem along with inhaled steroids can also result in iatrogenic CS.
The extent of steroid metabolism by the CYP3A4 isoenzyme is an important factor that determines the level of drug-drug interaction, when CYP3A4 inhibitors are co-administered. Other important properties that reduce the risk of drug–drug interaction include: 1) low systemic exposure because of the low glucocorticoid relative receptor binding affinity (RRA); 2) lower systemic oral bioavailability; 3) higher plasma protein binding; 4) shorter elimination half-life; 5) lower lipophilicity; 6) variation in CYP3A4 activity; 7) glucocorticoid receptor sensitivity; 8) glucocorticoid receptor polymorphism and 9) patients age.
Among the available ICS/INC, flunisolide and beclomethasone exhibit low RRA, short elimination half-life, low lipophilicity, and less dependence on CYP3A4 isozyme for its metabolism and fluticasone exhibits highest hypothalamic-pituitary-adrenal axis suppressive effect because of its pharmacokinetic properties, such as higher glucocorticoid RRA, higher lipophilicity, a longer elimination half-life and its high dependence for metabolism by CYP3A4.
CUSHING SYNDROME
Cushing syndrome (CS) is a group of clinical features caused by hypercortisolism [56]. CS takes its name from Harvey Cushing who, in 1912, first reported a patient with features of hypercorticolism [57]. CS can be due to endogenous causes such as pituitary tumour, ectopic ACTH production, adrenal tumour or exogenous causes like exogenous steroid administration. The term CS is used to describe the disease from all the causes, whereas Cushing’s disease is reserved for cases of pituitary-dependent CS. Exogenous glucocorticoid administration is the most common cause of CS. Endogenous CS is less common [58].
Depending upon the level of plasma ACTH, the causes of Cushing syndrome can be broadly divided into two: 1) ACTH dependent CS and 2) ACTH–independent CS. Cushing’s syndrome due to exogenous steroid administration is variably described as exogenous Cushing’s, steroid-induced Cushing’s or iatrogenic CS [59].
The pathophysiological mechanism varies depending upon the cause of CS [60]. In iatrogenic CS, the exogenous steroids suppress HPA axis resulting in decreased production of cortisol.
CLINICAL FEATURES OF CUSHING’S SYNDROME
Full blown CS is unmistakable clinically. But in mild and early cases, the clinical presentation is broad, and can be a diagnostic challenge. None of the clinical features of CS are pathognomonic, and many are nonspecific, adding to the diagnostic challenge.
The most common feature of Cushing's syndrome is progressive central (centripetal) obesity, which usually involves the face, neck, trunk, abdomen and, internally, spinal canal and mediastinum [61]. Fat accumulation, in the cheeks and in the temporal fossae results in "moon" face, in the back of neck results in "buffalo hump" or dorso-cervical fat pad and also enlarged fat pads fill the supra-clavicular fossae. But in pediatric cases, glucocorticoid excess may result in generalized obesity.
The characteristic dermatologic changes of CS are usually not seen in other similar conditions such as pseudo-Cushing’s [62]. Stretching of the fragile skin due to the enlarging trunk, breasts, and abdomen leads to development of broad, reddish-purple striae. The red-purple livid striae greater than 1 cm in width which is typical, and almost pathognomonic, are most commonly found over the abdomen, but are also present on the upper thighs, breasts, and arms.
Increased ACTH induces hyper-pigmentation, and it is not by excess cortisol. In humans ACTH is the principal hormone which induces pigmentation, and it acts via binding to melanocyte-stimulating hormone receptors [62]. Ectopic ACTH syndrome is most commonly associated hyper-pigmentation, where as pituitary overproduction of ACTH is less commonly associated, and those with adrenal tumours or iatrogenic Cushing syndrome is usually not associated with hyper-pigmentation, because of suppressed ACTH secretion.
Menstrual abnormalities are also common in CS [63]. Signs of androgen excess like increased libido, hirsutism, virilization [including temporal balding, deepening of the voice, male body habitus, male escutcheon, and clitoral hypertrophy], are most common in women with adrenal carcinoma compared to other causes of Cushing's syndrome [64]. Adrenal glands are the major source of androgens in women. Signs of androgen excess are usually not seen in men with Cushing's syndrome. In comparison, signs of androgen excess are more common in women with adrenal carcinoma, less common in women with ACTH-dependent CS, and not usually occur in women with adrenal adenomas.
Psychiatric abnormalities are seen in about 50% of patients with CS. Common psychiatric abnormalities include agitated depression, lethargy, paranoia, overt psychosis, insomnia, emotional lability, irritability, anxiety and panic attacks [65].
Proximal muscle wasting and weakness are common in CS, due to the catabolic effects of excess glucocorticoids on skeletal muscle, and are not seen in patients with pseudo-Cushing's syndrome [66]. The weakness in patients with CS is aggravated by associated hypokalemia which is due to increased mineralocorticoid activity.
Osteoporosis is common in patients with CS, which is caused by decreased absorption of calcium from the intestine, decreased bone formation, increased bone resorption, and decreased renal re-absorption of calcium [67]. Avascular necrosis of the femoral head occurs more commonly in patients with exogenous CS [68]. Low back pain is also very common. Glucose intolerance is common and frank diabetes can be seen in some cases, especially in patients with a family history of type 2 diabetes mellitus [69].
Hypertension is seen in up to 75% of cases. The mechanisms include mineralocorticoid effects of cortisol, the action of cortisol on peripheral and systemic vasculature, and activation of the renin-angiotensin system [70, 71]. Severe hypertension and hypokalemia are more prevalent in patients with CS secondary to ectopic ACTH [72]. Cardiovascular events are also more common in patients with CS, there by increasing the morbidity and mortality [73]. Patients with CS are more prone for infections, due to the inhibitory effects of glucocorticoids on the immune function. Increase in intraocular pressure, posterior sub-capsular cataract and retinal detachment are the ophthalmologic problems in CS [74].
The common clinical clues to differentiate ectopic ACTH secretion from the Cushing’s disease are male sex, atypical presentation, fast progression, severe myopathy, very high cortisol/ACTH values and severe hypokalemia. Few features of Cushing’s syndrome have more discriminatory value than others, which includes reddish purple striae, proximal muscle, weakness, plethora, bruising with no obvious trauma, and unexplained osteoporosis [75].
CLINICAL PRESENTATION OF CS IN PAEDIATRIC PATIENTS
Growth failure with associated gain in weight is one of the most important clinical features presents in paediatric CS [76]. Sleep disruption, muscular weakness, and problems with memory are less commonly seen in children’s with CS, compared to adults. In paediatric CS generalized obesity is seen, in contrast to adult CS which is associated with central obesity [77].
IATROGENIC CS
Iatrogenic CS is the most common cause of CS [78]. The development of CS depends on the dose, duration, and potency of the corticosteroids used in clinical practice. Exogenous CS presents with the same signs and symptoms as spontaneous CS. But some features, such as an increase in intraocular pressure, benign intracranial hypertension, cataracts, osteoporosis, aseptic necrosis of the femoral head, and pancreatitis, are more common in iatrogenic than endogenous CS, whereas features like hypertension, hirsutism, and oligomenorrhea/amenorrhea are less prevalent (Table 2) [79-86]. The clinical manifestations of iatrogenic CS are more striking than that of spontaneous Cushing’s, which occurs gradually. Generally, a Cushingoid appearance takes weeks or even months to develop depending upon the type and dose of the steroid used. However, it is difficult to predict doses and time courses at which CS will develop, because various factors like different potencies, different formulations, different modes of delivery of various glucocorticoids and varying levels of sensitivity of the individual patients to glucocorticoids, will complicate the issue.
Drug interaction is an important precipitating factor for iatrogenic CS, especially with co-administration of CYP450 inhibitors. Most of the case reports in patients using ICS are related to interaction with ritonavir, itraconazole, verapamil and diltiazem. Various case reports shows that in patients on ICS and PI, the duration of co-administration of corticosteroid with PI before the onset of symptoms ranges from 10 days to 5 years with a mean of 7.1 months [87, 88]. Clinical features reported includes 'Cushingoid facies' or 'moon face', dorso-cervical fat pad (also known as 'buffalo hump'), central obesity and weight gain, facial hirsutism, striae and easy bruising, most of them were common symptoms associated with Cushing's syndrome. A comparison of iatrogenic and spontaneous CS is given in the Table 2.
Co-administration of ICS and PI in HIV positive patients can be problematic because of the diagnostic confusion between iatrogenic CS and the antiretroviral-associated lipodystrophy, since their overlapping clinical features can lead to delayed diagnosis of iatrogenic CS [89]. However, rapid weight gain, increased appetite, abdominal striae, easy bruising, facial hirsutism, facial plethora, and neuropsychological manifestations are commonly associated with CS where as peripheral atrophy is more commonly associated with lipodystrophy. The distinguishing feature of HIV-associated lipodystrophy is that visceral fat deposition is accompanied by normal or decreased (but not increased) amounts of subcutaneous fat. Lipodystrophy is a slow, chronic change of fat distribution, usually evolving over many years, compared to rapid evolution in iatrogenic CS [90].
PSEUDO-CUSHING’S SYNDROME
Some patients will have mild to moderate hypercortisolism due to chronic over-activity of the HPA axis without true CS. So there may be hypercortisolism, abnormal dexamethasone suppressibility and mild elevation of urinary free cortisol (UFC), causing diagnostic confusion with true CS, and is called Pseudo-Cushing’s syndrome [91].
The test that helps to differentiate true from pseudo-Cushing’s syndrome is dexamethasone suppression test followed by a CRH stimulation test. Patients with pseudo-Cushing’s syndrome do not respond to CRH stimulation, while patients with true CS respond with a stimulated cortisol value of more than 1.4 μg/dL.
INVESTIGATIONS
In a case of suspected CS, the laboratory evaluation starts with the demonstration of hyper-cortisolism by showing increased and basal serum cortisol, 24 hours urinary free cortisol excretion, mid night salivary or serum cortisol and non-supressibility after an overnight dexamethosone suppression test. Once the hyper-cortisolism is confirmed, the next step is to measure plasma ACTH level to see whether the condition is ACTH-dependent CS, due to either pituitary tumours or ectopic ACTH secretion; or ACTH independent CS, due to basic adrenal pathology [92].
However, the diagnostic evaluation in patients with Iatrogenic CS shows entirely different results. The endocrine workup in iatrogenic CS shows a low 24-hour urine cortisol, a very low serum cortisol level and a suppressed plasma ACTH level. The most valuable laboratory test is the detection of synthetic glucocorticoids in the urine by high-pressure liquid chromatography [93, 94]. Reduction in the level of DHEAS after starting ICS/INS is found to be an early marker of adrenal suppression [95]. A diagnostic algorithm of CS is shown in Fig. (3).
TREATMENT
In Iatrogenic CS due to ICS/INS, the possible treatment options include: (1) use of an alternative medication instead of steroids, such as an anti-cholinergic agent (e.g. ipratropium), a mast cell stabilizer (e.g. cromolyn),a leukotriene receptor antagonist (e.g. montelukast), or an antihistamine (2) if drug interaction is suspected, substituting the inhaled/intranasal corticosteroid with corticosteroid of less potential for drug interactions (3) if drug interaction is suspected in HIV positive individuals on PI, based on the drug resistance and treatment history, change the PI to another antiretroviral agent without CYP3A4 isozyme inhibitory activity (such as nonnucleoside reverse transcriptase inhibitor, chemokinereceptor 5 (CCR5 receptor) antagonist or integrase inhibitors (specifically raltegravir or dolutegravir), if this is allowable or substituting the inhaled/intranasal corticosteroid with another corticosteroid with less potential for drug interactions and (4) use the lowest possible dose of ICS/INC along with CYP3A4 isozyme inhibitors, if both has to be continued depending upon the clinical condition [96].
In patients with iatrogenic CS, adrenal suppression is common and should be evaluated for the potential need to initiate oral steroid replacement and its gradual tapering [97]. Iatrogenic CS is usually associated with evidence of Adrenal insufficiency [98, 99].
PREVENTION OF IATROGENIC CS
The goal for using all inhaled and intranasal corticosteroids are to (1) produce long-lasting and potent therapeutic effects at the site of action, (2) lower systemic bio-availability, and (3) minimize systemic adverse effects by rapid elimination of the absorbed drug. The ideal inhalational should have the properties shown in the Table 3.
Inhaled or intranasal beclomethasone, budesonide and flunisolide are relatively safe and can be used in patients on CYP3A4 inhibitors. Based on the pharmacokinetic properties beclomethasone and budesonide, appear to be safer because of their lower binding affinity for glucocorticoid receptors and shorter elimination half-life, and flunisolide have a low risk of drug–drug interactions because of its low glucocorticoid RRA, low lipophilicity, weak CYP3A4 metabolism and short elimination half-life.
CONCLUSION
Uses of ICS/INS are associated with potential risk of development of Iatrogenic CS, especially with co-administration of CYP3A4 inhibitors. Clinical features of iatrogenic CS resemble that of spontaneous CS. In HIV infected patients on ICS/INS and PI, development of iatrogenic CS can be confused with anti-retroviral associated lipodystrophy, leading to delay in diagnosis. Cushingoid features with adrenal suppression and low serum cortisol are the features of iatrogenic CS. Reducing the dose of the steroids to minimum, substituting steroids with less potent steroids or with other alternative drugs and avoiding drug interactions are the various options for the prevention/ treatment of Iatrogenic CS, along with steroid supplementation and gradual tapering in case of associated adrenal insufficiency.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


