All published articles of this journal are available on ScienceDirect.
Assessment of the Non-Cystic Fibrosis Bronchiectasis Severity: The FACED Score vs the Bronchiectasis Severity Index
Abstract
Introduction :
Non-cystic fibrosis bronchiectasis (NCFB) is a multidimensional disease, and no single isolated parameter is proved to have sufficient power for any overall determination of its severity and prognosis.
Objective :
To compare the results of the assessment of the NCFB severity with respect to its prognosis in the same patients by two different validated scores, i.e. the FACED score and the Bronchiectasis Severity Index (BSI).
Methods :
An observational study including 37 patients with NCFB (16 males and 21 female aged 46 to 76 years) was performed. All patients underwent evaluation of the variables incorporated in the FACED score (FEV1 % predicted, age, chronic colonization by Pseudomaonas aeruginosa, radiological extent of the disease, and dyspnea) and in the BSI (age, body mass index, FEV1 % predicted, hospitalization and exacerbations in previous year, dyspnea, chronic colonization by Pseudomaonas aeruginosa and other microrganisms, and radiological extent of the disease).
Results :
According to the value of the derived overall FACED score we found 17 patients (45.9%) with mild bronchiectasis, 14 patients (37.8%) with moderate bronchiectasis and 6 patients (16.2%) with severe bronchiectasis. The mean derived FACED score was 3.4 ± 1.3. In addition, according to the value of the derived overall BSI score, the frequency of patients with low, intermediate and high BSI score was 16 patients (43,2%), 14 patients (37.8%) and 7 patients (18.9%), respectively. The mean derived BSI score was 6.4 ± 2.5.
Conclusion :
We found similar results by the assessment of the NCFB severity in regard to its prognosis by both the FACED score and the BSI. Further studies determining how these scores may impact clinical practice are needed.
INTRODUCTION
Bronchiectasis is considered as a heterogeneous condition characterized by irreversible airway dilatation with chronic bronchial infection/inflammation. This is pathological description of a disease process that has many possible causes [1, 2].
Bronchiectasis is generally classified into cystic fibrosis and non-cystic fibrosis bronchiectasis (NCFB). NCFB may result from a number of congenital and acquired causes, with the later more frequent. Congenital causes include primary ciliary dyskinesia, primary immunodeficiencies, situs inversus, etc. Tuberculosis, pneumonia, inhaled foreign bodies, pulmo-nary aspiration, corticosteroid-dependent asthma, allergic bronchopulmonal aspergillosis and bronchial tumours are the major acquired causes of NCFB [3,4]. Infective causes associated with NCFB include infections caused by Staphylococcus aureus, Klebsiella, Bordetella pertusis, etc. Various immunological disorders, such as childhood acquired immune deficiency syndrome (AIDS), inflammatory bowel disease (especially ulcerative colitis) and rheumatoid arthritis, are also linked to the development of NCFB [5, 6]. In a significant number of cases the underlying etiology of NCFB remains unidentified and is subsequently referred to as idiopathic [7]. In addition, there is evidence that 50% of patients with chronic obstructive pulmonary disease (COPD) have co-existent bronchiectasis [8].
NCBF is a common condition. According to the actual estimation, there are at least 110,000 adults in the USA with this condition causing economic costs at 630 million dollars per year [9, 10]. As it is recognized that its prevalence increases around the world, bronchiectasis became an important public health problem [6]. With respect to this, there was a need for a predictive tool for assessment of the disease severity in routine practice which would allow targeting of therapies to the patients most likely to benefit and improving their quality of life [11]. In the recent years, two multidimensional grading systems capable of classifying the severity of bronchiectasis according to its prognosis were designed: the FACED score and Bronchiectasis Severity Index (BSI). The FACED score (forced expiratory volume in 1 second (FEV1) % predicted [F], age [A], chronic colonization by Pseudomonas aeruginosa [C], extension of the disease by radiological assessment [E] and dyspnea [D]) [12] is a five-point score that predicts probability of all-cause mortality after 5 years of follow-up, whereas the BSI [13] is a seven-point score that identifies patients with NCFB at risk for future mortality, hospitalization and exacerbations.
The present study aims to compare the results of the assessment of NCBH severity in the same patients done by the FACED score and by the BSI.
MATERIALS AND METHODOLOGY
Study Design and Setting
An observational study, i.e. comparison between NCFB severity scores derived in the same patients by the FACED score and the BSI, was performed at the Institute for Occupational Health of R. Macedonia, Skopje - WHO Collaborating Center and GA2LEN Collaborating Center in the period May-November 2014.
Patients
We examined 37 patients (16 males and 21 females) aged 46 to 76 years with stable bronchiectasis. The diagnosis was established according to the actual recommendations, i.e. by high-resolution computed tomography (HRCT) of the chest in the subjects with clinical presentation consistent to bronchiectasis [14-16].
Inclusion criteria were: clinically stable patients with NCFB with no antibiotic use in the preceding 4 weeks.
Exclusion criteria were: active malignancy, cystic fibrosis (CF), active mycobacterial disease, human immunodeficiency virus (HIV) infection, pulmonary fibrosis with secondary traction bronchiectasis, and treatment with a long-term antibiotic therapy.
All participants were informed about the study and their written consent was obtained.
The FACED Score
The FACED score incorporates 5 dichotomised variables:
- FEV1 % predicted (cut-off 50%, maximum value 2 points),
- Age (cut-off 70 years, maximum value 2 points),
- Presence of chronic colonization by Pseudomonas aeruginosa (dichotomic, maximum value 1 point),
- Radiological extension (number of lobes affected, cut-off 2 lobes, maximum value 1 point)
- Dyspnea (cut-off grade II on the Medical Research Council [MRC] scale, maximum value 1 point).
An overall score is derived as a sum of the scores for each variable and it may range from 0 to 7 points. By this score, the bronchiectasis is classified into 3 severity classes: mild bronchiectasis (overall score 0-2 points), moderate bronchiectasis (overall score 3-4 points) and severe bronchiectasis (overall score 5-7 points) (12).
BSI
The BSI incorporates 9 variables:
- Age: less than 50 years (0 points); 50-69 years (2 points), 70-79 years (4 points), more than 80 years (6 points)
- Body mass index (BMI): less than 18.5 (2 points), more than 18.5 (0 points)
- FEV1 % predicted: less than 80% (0 points), 50-80% (1 point), 30-49% (2 points), less than 30% (3 points)
- Hospital admission in previous year: no (0 points), yes (5 points)
- Exacerbations in previous year: 0-2 (0 points), 3 or more (2 points)
- MRC dyspnea score: 1-3 (0 points), 4 (2 points), 5 (3 points)
- Pseudomonas aeruginosa colonization: no (0 point), yes (3 points)
- Colonization with other microorganisms: no (0 point), yes (1 point)
- Radiological severity (more than 3 lobes involved or cystic bronchiectasis): no (0 points), yes (1 point)
An overall score is derived as a sum of the scores for each variable and it may range from 0 to 26 points According to the overall score value, the patients with bronchiectasis are classified into 3 BSI classes: patients with low BSI score (overall score 0-4 points), patients with intermediate BSI score (overall score 5-8 points) and patients with high BSI score (overall score 9 or more points) [13].
Patient Analysis
At the time of clinical assessment all patients were clinically stable, with no antibiotic use in the preceding 4 weeks. Classification of smoking status was done according to the World Health Organization (WHO) guidelines on definitions of smoking status [17]. Pack-years smoked (one pack-year denotes one year of smoking 20 cigarettes per day) were calculated according to the actual recommendations [18].
The severity of dyspnea was graduated according to the MRC breathlessness scale into 5 grades: grade 1 (patient is not troubled by breathlessness except on strenuous exercise), grade 2 (getting short breath when hurrying on the level or walking up a slight hill), grade 3 (walking slower than most people on the level, stopping after a mile or so, or stopping after 15 minutes walking at own pace), grade 4 (stopping for breath after walking about 100 yds or after a few minutes on level ground) and grade 5 (being too breathless to leave the house or being breathless when undressing) [19].
All patients underwent spirometry including measures of forced vital capacity (FVC) and FEV1 with recording the best result from three measurements the values of which were within 5% of each other. The results of spirometry were expressed as percentages of the predicted values according to the actual recommendations of European Repsiratory Society (ERS) and American Thoracic Society (ATS) [20, 21].
Bacteriological status of the patients was assessed on spontaneous early-morning sputum samples. Chronic colonization was considered by isolation of potentially pathogenic bacteria in sputum culture on at least two occasions in a period of 3 months [22] as the predominant pathogen underwent bacterial growth most frequently over this period of time. Patients who were unable to provide sputum samples (e.g. due to absence of productive cough) were classified as non-colonized.
Radiological evaluation, i.e. the extent of bronchiectasis, was made according to the number of pulmonary lobes affected (with the lingula considered as independent lobe) and the degree of bronchial dilatation (tubular, varicose or cystic). A small bronchiectasis visible only in a single pulmonary segment was not considered, as this can appear in a significant proportion of the healthy population [15, 23].
Statistical Analysis
Data for quantitative variables were expressed as mean value with standard deviation (SD), while the frequencies variables were expressed as absolute values and the percentage of the total.
RESULTS
Characteristics of the patients enrolled in the study are shown Table 1.
Characteristics of the study participants.
| Characteristics | Patients (n = 37) |
|---|---|
| M/F ratio Mean age (yrs) BMI (kg.m-2) Smoking status Daily smokers Pack-years smoked Ex-smokers Dyspnea (MRC score) Sputum production Mean FVC value (% pred.) Mean FEV1 value (% pred.) Chronic colonisation Pseudomonas aeruginosa Other microorganisms Number of lobes affected Exacerbation in previous year Hospitalization in previous year Chronic treatment in previous year Systemic antibiotics Macrolides Oral corticosteroids |
0.8 63.4 ± 8.1 24.3 ± 3.7 14 (37.8%) 12.8 ± 5.7 5 (13.5%) 1.83 ± 0.63 29 (78.4%) 73.8 ± 11.4 57.6 ± 8.7 3 (8.1%) 6 (16.3%) 2. 25 ± 0.78 2.12 ± 0.54 1.14 ± 0.37 8 (21.6%) 3 (8.1%) 6 (16.3%) |
Numerical data are expressed as a mean value with standard deviation; the frequencies as a number and percentage of patients with certain variable
M: male; F: female; yrs: years; BMI: body mass index; kg: kilogram; m: meter; MRC: Medical Research Council; FVC: forced vital capacity; FEV1: forced expiratory volume in 1 second; % pred.: % of predicted value.
Values of the FACED score variables.
| Variable | Patients (n = 37) |
|---|---|
| FEV1 % predicted < 50% > 50% Age (yrs) > 70 < 70 Chronic colonization by Pseudomonas aeruginosa Yes No Radiological extent (HRCT) > 2 lobes affected < 2 lobes affected MRC dyspnea score > 2 (II) < 2 (II) |
9 (24.3%) 28 (75.7%) 7 (18.9%) 30 (81.1%) 3 (8.1%) 34 (91.9%) 16 (43.2%) 21 (56.8%) 17 (45.9%) 20 (54.1%) |
Frequencies are expressed as a number and percentage of patients with certain variable.
FEV1: forced expiratory volume in 1 second; yrs: years; HRCT: high-resolution computed tomography; MRC: Medical Research Council.
Values of the BSI variables.
| Variable | Patients (n = 37) |
|---|---|
| Age (yrs) < 50 50-69 70-79 > 80 BMI (kg.m-2) < 18.5 > 18.5 FEV1 % predicted > 80% 50-80% 30-49% < 30% Hospital admission in previous year No Yes Exacerbations in previous year No Yes MRC dyspnea score 1-3 4 5 Pseudomonas aeruginosa colonization No Yes Colonization with other microorganisms No Yes Radiological extent (HRCT) > 3 lobes affected or cystic bronchiectasis No Yes |
8 (21.6%) 22 (59.4%) 7 (18.9%) / 2 (5.4%) 35 (94.6%) 5 (13.5%) 23 (62.2%) 9 (24.3%) / 6 (16.2%) 31 (83.8%) 21 (56.7%) 16 (43.3%) 33 (89.2%) 4 (10.8%) / 34 (91.9%) 3 (8.1%) 31 (83.7%) 6 (16.3%) 27 (73.0%) 10 (27.0%) |
Frequencies are expressed as a number and percentage of patients with certain variable.
Yrs: years; BMI: body mass index; kg: kilogram; m: meter; FEV1: forced expiratory volume in 1 second; MRC: Medical Research Council; HRCT: high-resolution computed tomography.
The values of the FACED score variables are presented on Table 2.
According to the value of the derived overall score we found 17 patients (45.9%) with mild bronchiectasis, 14 patients (37.8%) with moderate bronchiectasis and 6 patients (16.2%) with severe bronchiectasis (Fig. 1). The mean derived FACED score was 3.4 ± 1.3.

Distribution of the patients by the FACED score.
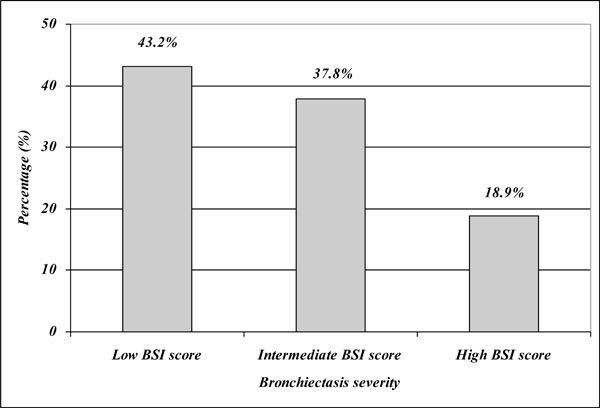
Distribution of the patients by the BSI score.
The values of the BSI variables are presented on Table 3.
According to the value of the derived BSI score we found 16 patients (43.2%) with low BSI score, 14 patients (37.8%) with intermediate BSI score and 7 patients (18.9%) with high BSI score (Fig. 2). The mean derived BSI score was 6.4 ± 2.5.
DISCUSSION
NCFB is a chronic progressive condition resulting from infection and inflammation of the airway leading to destruction and remodeling of the bronchial wall [6, 24]. The clinical manifestations of the disease include chronic and commonly purulent expectoration, multiple exacerbations and progressive dyspnea. All these events cause progressive decline in the lung function and impairment of the quality of life of the patients [25, 26]. As with other airway diseases (e.g. with COPD), the severity and prognosis of bronchiectasis can not sufficiently be assessed by one single parameter, so multidimensional approach to this issue is required. Most respiratory diseases have a disease-specific severity assessment tool. The severity assessment is needed for appropriate treatment of the patients, stratification of the risk of complications, reduction of the costs associated with particular disease, as well as for researching activities (i.e. identification groups of patients likely to benefit from novel therapies) [5, 27, 28] .
Up to recent years there was no any scoring system for assessment of the NCFB severity and prognosis, and now there are two scoring systems designed for this purpose, the FACED score and the BSI. These scores have different structure and somewhat different aims. The FACED score is easy-to-use tool incorporating 5 dichotomic variables, whereas the BSI is relatively complex, awarding different point values for each of the variables and including multiple variables. In addition, the FACED score is aimed at prediction of the probability of all-cause mortality of the patients with NCFB in the next 5 years, whereas the BSI is aimed at identification of the patients with NCFB with higher risk for future mortality, hospitalization and exacerbations [12, 13].
In the present study we compared the results from the assessment of severity of the disease in patients with NCFB by these two scoring systems. The study group included patients with stable NCFB of both sexes aged 46 to 76 years. The study work-up consisted of evaluation of each variable incorporated in the FACED score and in the BSI. The scores derived by both scoring systems were similar. Namely, by the FACED score we found that the frequency of mild, moderate and severe bronchiectasis was approximately 46%, 38% and 16%, respectively. According to the score derived by the BSI, we found that the frequency of patients with low, intermediate and high BSI was 43%, 38% and 19%, respectively. As in the available literature we did not find similar study, we can not compare the results obtained in our study with results of other studies. Taking into account the public health aspects of bronchiectasis, we expect that further studies for more precise determining the role of these scoring systems in the clinical practice and research area will be performed soon.
The present study has some limitations. Firstly, relatively small number of the subjects in the study group could have certain implications on the data obtained and its interpretation. Secondly, the majority of the patients in our study suffered from idiopathic and post-infective bronchiectasis, so the study is not powered to detect anything other than very large effects on survival in bronchiectasis due to less common etiologies (e.g. bronchiectasis in patients with systemic diseases). The strength of the study is the comparison of the results of two different predictive tools for assessment of the severity of the NCFB with respect to its prognosis.
In conclusion, in an observational study aimed at comparison of the results of the NCFB severity assessment with respect to its prognosis similar results were obtained by both the FACED score and the BSI. Our findings indicate that both scores can be used in routine clinical work, but there is a need for further studies in order to determine how these scores may impact the clinical practice.
AUTHORS PARTICIPATIONS
JM participated in the study design, writing the protocol, data collection, managing the analyses of the study, and writing all versions of the manuscript. JKB participated in the study design, writing the protocol, managing the analyses of the study, as well as writing all versions of the manuscript. KV participated in the study design and in the managing of the analyses of the study. SS and DM participated in the data collection and in the managing of the analyses of the study. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


