All published articles of this journal are available on ScienceDirect.
Gulf Asthma Diagnosis and Management in Adults: Expert Review and Recommendations
Abstract
The prevalence and incidence of asthma are increasing globally because of genetic and environmental influences. Prevalence of asthma in the Gulf has been reported to range from 4.7% to 32.0% and has a substantial economic burden. In this paper, we summarize current asthma management guidance for adults, present insights, and recommendations by key opinion leaders (KOLs) in the Gulf region, and key performance indicators for guiding clinical practice for asthma diagnosis, management, and treatment in the Gulf. While it is recommended that the Global Initiative for Asthma (GINA) guidelines should be followed wherever possible for the management of asthma, KOLs in the Gulf region have presented additional recommendations based on regional challenges and insights. There is a need for better diagnosis using objective testing, increased efforts in tackling the burden of comorbidities in the region, and greater provision of the necessary tools for phenotyping severe asthma. Furthermore, there is a need for greater education for physicians regarding asthma treatment, including the importance of inhaled-corticosteroid-containing controller medication. Regionally, there is also a need for specialist asthma clinics and asthma educators, which would serve to educate physicians and their patients as well as to improve the management of patients. Finally, the use of asthma registries, digital devices, and electronic templates would be of benefit in the management of asthma patients in the region.
1. INTRODUCTION
1.1. Asthma and Global Epidemiology
Asthma is an inflammatory condition of the airways that clinically manifests as dyspnea, wheezing, chest tightness, and coughing [1]. The disease is variable since symptoms can change with time and in severity [2]. Asthmatic obstruction of the airways can be caused by the release of inflammatory cells and changes to the respiratory epithelium which can be life-threatening [1, 3].
Asthma is a heterogeneous disease with different phenotypes, of which the eosinophilic phenotype is one of the most well-recognized [4]. The eosinophilic phenotype involves T-helper cell type 2 (Th2) and innate lymphoid cell activation, which leads to abnormal production of type 2 cytokines, namely Interleukin (IL)-4, IL-5 and IL-13 [5]. Treatments targeting IL-5 (i.e., anti-IL-5 monoclonal antibodies) hold a prominent role in asthma management since IL-5 binds the alpha chain of IL-5 receptor and regulates eosinophil promotion, migration, maturation, and survival [6]. Interestingly, upon IL-5 activation, eosinophils degranulate and release cytotoxins with antimicrobial effects inducing damage to surrounding cells and tissue [7]. In addition, the production of immunoglobulin E (IgE) is stimulated [1, 3]. IgE binds to receptors on the surface of mast cells and eosinophils, activating the cells and resulting in obstruction of the airways [1]. As well as their role in the Th2 reaction, IL-4 and IL-13 cytokines are also involved in epithelial cell function and contribute to the chronicity and severity of asthma [8]. Thymic stromal lymphopoietin is a tissue-derived cytokine that induces the production of chemokines such as thymus- and activation-regulated chemokine (TARC/CCL17), which in turn stimulate Th2 development [9]. Type 2 innate lymphoid cells also play a role in type 2 airway inflammation [10]. Recently it has been suggested that Th17 and Th9 also contribute to the inflammatory process [11]. Th9 cells produce IL-9, which is associated with mast cell and eosinophil recruitment to the lung as well as the over-secretion of mucus [12]. Th17 cells produce IL-17, which plays a role in neutrophil- and macrophage-associated inflammation of the lung [13]. IL-17A and IL-17F have also been associated with severe and resistant types of asthma [13].
In 2015, there were an estimated 358 million cases of asthma globally, which resulted in 397,000 deaths [14]. The prevalence and incidence of asthma are considered to increase resulting from both genetic and environmental factors and are predicted to increase by approximately 100 million by 2050 [15, 16]. Such an increase is likely to pose an even greater burden on healthcare systems.
The economic burden of asthma is substantial, resulting from direct costs such as hospitalization, emergency visits, and treatments, as well as indirect costs such as loss of working hours, loss of education hours, and travel time to healthcare facilities [17]. Asthma severity and the level of patients’ symptom influence the utilization of resources (e.g., hospital/ clinic care and medication) which impact overall healthcare costs [18]. Factors including comorbidities, age, sex, ethnicity and pharmacogenomic variability can also impact the cost (i.e., economic burden) of asthma [17]. Pharmacogenomic variability causes significant heterogeneity in asthma treatment outcomes [19]. For example, approximately 10% of asthmatic patients are refractory to treatment and evidence for genetic variations associated with poor treatment response has been identified for multiple groups of drugs (and genes) such as inhaled corticosteroids (FCER2), anti-leukotriene agents (ABCC1 and LTC4S); beta-agonists (ADRB2) [20]. Genetic variations in the expression of drug targets and/or enzymes involved in drug biodisposition may significantly impact a patients ability to achieve adequate symptom control and therefore the overall economic burden of asthma [21, 22].
1.2. Local Burden and Cost of Asthma
Studies have reported varying prevalence rates of asthma across the Gulf, including Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates (UAE) (Table 1). Such studies have generally focused on small populations within a single country; however, larger studies have been conducted across a number of countries, including the Asthma Insights and Reality in the Gulf and the Near East (AIRGNE) [23] and the Asthma Insights and Management in the Gulf and Russia (AIM) [24] studies. Prevalence of asthma has been reported to range from 4.7% to 32.0%. Much of these data were derived without the use of stratified population sampling, which should be used to account for the distribution of nationals and non-nationals within these populations; therefore, the reported prevalence may not reflect the true prevalence within the population. Furthermore, there is a lack of data concerning asthma-associated mortality in the Gulf, with only one published study to date: it reported a mortality rate of 0.9 per 100,000 in Kuwait in 2000 [25]. There is a lack of data regarding severe asthma in the region. In Oman, the prevalence of severe asthma symptoms (including speech-limiting wheeze, sleep disturbance, or wheezing attacks) was reported in 2001 to be 5.1% in children aged 6–7 years and 5.3% in children aged 13–14 years [26].
| Country | Prevalence (%) | Age (Years) | Method | Month / Year | Study / Reference |
|---|---|---|---|---|---|
| Bahrain | 11.3 | 6–7 | International Study of Asthma and Allergies in Childhood (ISAAC) | June 2010–July 2010 | Al-Sindi et al. 2014 [27] |
| Kuwait | 23.0 | Adults (mean 39) & children (mean 8) | AIR study questionnaire, ATC, and additional questions | January 2007–March 2008 | AIRGNE [23] |
| Kuwait | 8.5 | ≥12 | AIM questionnaire | December 2016–February 2017 | AIM [24] |
| Kuwait | 15.6 | 13–14 | ISAAC | 2001–2002 | Owayed et al. 2008 [28] |
| Oman | 10.5 20.7 |
6–7 13–14 |
ISAAC | 1995 | Al-Riyami et al. 2003 [29] |
| Oman | 10.6 19.8 |
6–7 13–14 |
ISAAC | 2001 | Al-Rawas et al. 2008 [26] |
| Oman | 27.0 | Adults (mean 39) & children (mean 8) | AIR study questionnaire, ACT, and additional questions | January 2007–March 2008 | AIRGNE [23] |
| Oman | 7.8 8.9 |
6–7 13–14 |
ISAAC (any wheeze) | Not reported | Al-Riyami et al. 2001 [30] |
| Qatar | 19.8 | 6–14 | ISAAC | February 2003–February 2004 | Janahi et al. 2006 [31] |
| Saudi Arabia | 8.0 23.0 |
8–16 | Standardized questionnaire | 1986 1995 |
Al Frayh et al. 2001 [32] |
| Saudi Arabia | 19.6 | 16–18 | ISAAC | 2009–2010 | Al Ghobain et al. 2012 [33] |
| Saudi Arabia | 11.3 | 20–44 | ECRHS | April 2016–June 2016 | Al Ghobain et al. 2018 [34] |
| Saudi Arabia | 4.7 | ≥12 | AIM questionnaire | December 2016–February 2017 | AIM [24] |
| UAE | 13.6 | 6–14 | Standardized questionnaire | October 1992–May 1993 | Bener et al. 1994 [35] |
| UAE | 12.1 | Mean 32.9 | ECRHS | January 2010–March 2010 | Mahboub et al. 2012 [36] |
| UAE | 13.0 | 6–13 | ISAAC | Not reported | al-Maskari et al. 2000 [37] |
| UAE (Al Ain) | 12.0 | Median 30 | ISAAC | November 2007–January 2008 | Alsowaidi et al. 2008 [38] |
| UAE | 30.0 | Adults (mean 39) & children (mean 8) | AIR study questionnaire, ACT, and additional questions | January 2007–March 2008 | AIRGNE [23] |
| UAE | 8.5 | ≥ 12 | AIM questionnaire | December 2016–February 2017 | AIM [24] |
| Country | Outpatient Costs (%) | Inpatient Stays (%) | Emergency Visits (%) | Medication (%) | Total Direct Costs (USD) | Year | Reference |
|---|---|---|---|---|---|---|---|
| Kuwait | 20.8 | 43.2 | 29.1 | 6.9 | 208,244,564 | 2005 | Khadadah 2013 [39] |
| Oman | 19.4 | 25.0 | 55.3 | 0.3 | 159,900,761 | 2009 | Al-Busaidi et al. 2013 [40] |
| UAE (Dubai) |
37.0 | 27.0 | 20.0 | 16.0 | 23,956,293* | 2009 | Mahboub et al. 2013 [41] |
| UAE (Abu Dhabi) |
45.7 | 8.3 | 4.1 | 30.9 | 28,555,496* | 2011 | Alzaabi et al. 2014 [42] |
High expenditures of asthma have been reported in the Gulf, specifically in Kuwait, Oman, and the UAE (Table 2). However, these data are from studies conducted between 2005 and 2011. Therefore, there is a need for an evaluation of more recent asthma costs in the region. Regardless, the data show a large economic burden of asthma in the Gulf, with annual costs ranging from 23 million to 208 million US dollars per year. Some countries, in particular Kuwait and Oman, spend a large proportion of their costs on inpatient and emergency visits and a very low proportion of costs on medication; this may indicate that asthma is not being well managed or treated, with poor levels of asthma control.
1.3. Rationale
Asthma guidelines have been implemented globally to decrease the burden associated with asthma [43]. Such guidelines include the Global Initiative for Asthma (GINA) guidelines [44] as well as national guidelines such as those implemented in the United Kingdom [45], Australia [46], New Zealand [47], and the United States of America [48], among others. Not only do such guidelines serve to guide practicing physicians on the diagnosis, management, and treatment of asthma, they also inform patients, educate healthcare professionals, and inform regulatory agencies and policy makers [43]. Currently there are published asthma guidelines for only two Gulf countries, Saudi Arabia [49] and Qatar [50]. In this paper, we present insights and recommendations from key opinion leaders in the Gulf region as well as key performance indicators for guiding clinical practices for asthma diagnosis, management, and treatment in the Gulf.
2. CURRENT ASTHMA MANAGEMENT GUIDANCE
2.1. Asthma Assessment and Diagnosis
2.1.1. Diagnosis
According to GINA, the diagnosis of asthma requires two elements: (1) a history of respiratory symptoms that are typical of asthma and variable in nature, and (2) diagnostic evidence of variable expiratory airflow limitation [44]. Documentation of the basis of an asthma diagnosis is essential.
Upon suspicion of asthma, according to a patient’s clinical history, diagnosis is commonly established via spirometry. Peak expiratory flow (PEF), bronchodilator response (BDR) and/or confirmatory methacholine challenge testing (MCT) are important assessments for the diagnosis of asthma [44, 45, 51]. Interestingly, in a large real-world cohort of patients with physician-diagnosed asthma, Selvanathan et al. identified that the negative predictive value of spirometry with BDR was low for excluding asthma. The authors also reported that the presence of airflow limitation (i.e., a reduced ratio of the first forced expiratory volume [FEV1] to forced vital capacity [FVC]) predicted a positive MCT. Even in cases demonstrating a negative BDR, the presence of airflow limitation remains suggestive of asthma. Frequently repeating spirometric tests is recommended in patients with a high clinical suspicion of asthma since spirometric values may vary due to symptom fluctuation over time [2]. Further, asthma patients with near-normal spirometry or severe asthma may not show bronchodilator reversibility during BDR assessment due to airway re-modelling [52]. Notably, patients with chronic obstructive pulmonary disease (COPD) have demonstrated significant reversibility [53]. Therefore, asthma diagnosis via spirometry in isolation is not recommended since spirometry has limited value in differentiating asthma from COPD [54].
Symptoms of asthma usually include dyspnea, wheezing, chest tightness, and coughing [44]. Since such symptoms are variable in severity over time, the British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN) guidelines recommend comparing results from any diagnostic tests in both symptomatic and non-symptomatic states to capture such variation [45]. The BTS/SIGN guidelines highlight that, in practice, a diagnosis should involve identifying the signs, a distinctive pattern of respiratory symptoms, and the results of diagnostic tests as well as ruling out any alternative diagnoses [45].
2.1.2. Treatment Initiation and Stepwise Approach
Following diagnosis, patients should be initiated on a medication containing inhaled corticosteroid (ICS), starting with a low dose and moving to higher doses should symptoms persist [44]. GINA recommends a stepwise approach to adjusting a patient’s treatment [44].
2.1.3. Assessment
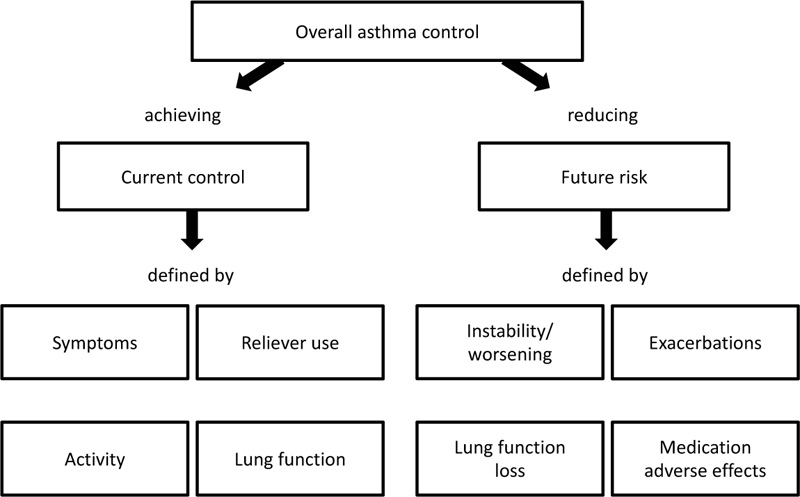
An asthma patient should be assessed at least once every year during symptomatic episodes, in the event of an exacerbation, or upon refill of an asthma medication prescription [44]. Routine clinical tests include repeated spirometry with PEF, BDR and/or MCT, as well as fractional exhaled nitric oxide (FeNO) which provides a noninvasive marker of inflammation in asthmatic patients [55]. According to GINA, there are three areas that should be covered at each assessment: (1) control of asthma symptoms and risk factors, (2) comorbidities, and (3) any issues surrounding treatment [44]. Control of asthma symptoms is essential to prevent exacerbations, treatment side effects, and loss of lung function. Objective assessment of asthma control can be undertaken via the use of validated questionnaires such as the Asthma Control Test (ACT) or the Asthma Control Questionnaire (ACQ) [44, 45]. Alternatively, the GINA asthma symptom control assessment includes four questions concerning the patient’s experience of any of the following in the previous 4 weeks: (1) daytime asthma symptoms more than twice a week, (2) any nighttime waking due to asthma, (3) short-acting β2-agonist (SABA) reliever for symptoms more than twice a week, and (4) any activity limitation due to asthma. Patients responding ‘No’ to all four questions are deemed to have well-controlled asthma, those responding ‘Yes’ to one or two questions are deemed to have partly controlled asthma, and those responding ‘Yes’ to three or four questions are deemed to have uncontrolled asthma [56]. The BTS/SIGN guidelines highlight the importance of identifying comorbidities that might place the patient at increased risk of exacerbations, including female sex, increased age, depression, smoking, obesity, and reduced lung function [45]. BTS/SIGN also highlights the need to assess the history of asthma exacerbations and review reliever use in addition to assessing asthma control [45]. Furthermore, asthma education is an important aspect to cover in all assessments. Ideally, lung function should be assessed at the start of treatment in addition to 3–6 months after the start of treatment [44].
In adults, asthma severity can be assessed based on the level of treatment that is able to control the symptoms as per GINA guidelines. Mild asthma is controllable using only as-needed ICS-formoterol or low-dose ICS, LTRA or chromones, whereas severe asthma requires high-dose ICS with long-acting β2-agonist (LABA) [44]. Assessment of acute asthma exacerbation severity according to BTS/SIGN is undertaken via an assessment of clinical signs and symptoms, PEF, vital signs, oxygen saturation, and, if required, blood gas analysis [45].
2.2. Asthma Management
2.2.1. Management Principles
The main goals of asthma management according to GINA should be controlling patient symptoms and reducing the risks associated with uncontrolled asthma [44]. Owing to the variable nature of asthma, a stepwise approach to treatment is recommended. Patients are initiated on the treatment step which is best suited to their symptoms (i.e., patients with more frequent symptoms and greater risks may not be initiated on treatment step one). A step-up in controller treatment and symptom management may be required when symptoms or exacerbations continue to be problematic, followed by a step down once symptom control has been established [44]. This stepwise approach requires routine assessment of the patient’s asthma [57] (Fig. 1).
Treatment options include pharmacological and non-pharmacological interventions and should focus on reducing/ treating risk factors and triggers associated with asthma such as depression, smoking, obesity, and reduced lung function [44, 45]. Pharmacological treatment, including an ICS-containing medication, should be used by all asthma patients [44]. ICS is the cornerstone of asthma management, with low doses being demonstrated to reduce hospitalization and deaths [59, 60], prevent severe exacerbations [61], improve lung function [62], reduce the symptoms of asthma [63], and reduce the frequency and severity of exercise-induced asthma [44, 64]. Leukotriene receptor antagonists (LTRA) are a further controller option, but these are less effective than ICS [65]. SABA alone is no longer recommended for the treatment of asthma [44]. All patients with asthma should have a reliever inhaler; however, if their asthma is well controlled, there should be little or no requirement for reliever medication [44].
The BTS/SIGN guidelines highlight the importance of self-management, with the support of healthcare professionals, in order to recognize and act swiftly upon presentation of any symptoms and signals of deteriorating condition [45]. Such self-management requires educating the patient via the provision of information regarding their condition as well as a written asthma action plan [44, 45].
2.2.2. Mild Asthma
Asthma that controlled by step 1 or step 2 of treatment is classified as mild, according to GINA [44]. Mild asthma is treated with low-dose ICS-formoterol as needed or low-dose ICS plus SABA. In patients with mild asthma, the SYGMA 1 trial demonstrated budesonide-formoterol to result in 65% fewer severe exacerbations versus treatment with the SABA terbutaline alone [66]. In the SYGMA 2 trial, budesonide-formoterol, as needed, was shown to be non-inferior to budesonide maintenance therapy in terms of annualized rate of severe exacerbations (0.11 vs 0.12 respectively) in patients suffering from mild asthma but inferior in terms of symptom control [67]. Additionally, an open-label trial in adults with mild asthma demonstrated superiority of budesonide-formoterol as needed versus albuterol as needed [68]. A further open-label trial in adults with mild to moderate asthma demonstrated that budesonide-formoterol as needed resulted in 31% fewer exacerbations than maintenance budesonide plus terbutaline as needed [69]. SABA alone is no longer recommended for treatment of mild asthma [44], as even patients with mild asthma can experience severe or even fatal exacerbations [70].
2.2.3. Moderate Asthma
Moderate asthma is asthma controlled by step 3 of the stepwise treatment approach [44]. At step 3, low-dose ICS-LABA is the preferred controller option; low-dose ICS-formoterol can be prescribed as needed as maintenance and reliever therapy [44]. The ICS-formoterol maintenance and reliever approach (budesonide-formoterol maintenance and reliever) have been shown to decrease the rate and risk of severe exacerbations of asthma, ameliorate the symptoms of asthma, reduce the frequency of nighttime waking, and improve lung function compared with budesonide plus SABA and budesonide-formoterol plus SABA [71]. Furthermore, budesonide/formoterol maintenance and reliever treatment has been shown to reduce the number of severe exacerbations compared with salmeterol/fluticasone plus SABA as-needed and higher fixed doses of budesonide/formoterol, with reduced hospitalization or treatment in the emergency room (ER) [72].
3. MANAGEMENT OF DIFFICULT-TO-TREAT AND SEVERE ASTHMA
3.1. Definitions
Uncontrolled asthma involves poor control of symptoms and/or frequent exacerbations [73]. Difficult-to-treat asthma is asthma that remains uncontrolled following step 4 or 5 of treatment [73]. Severe asthma is a subtype of difficult-to-treat asthma and remains uncontrolled despite following step 4 or 5 of treatment and despite confirmation of treatment adherence, correct inhaler technique, identification of risk factors and control of co-morbidities [73].
3.2. Treatment
The recommended controller at step 4 is medium-dose ICS/LABA; low-dose ICS-formoterol can be prescribed as needed as maintenance and reliever therapy [44]. According to the GINA guidelines for severe and difficult-to-treat asthma, patients diagnosed with difficult-to-treat asthma should first have their diagnosis confirmed, followed by the assessment of any factors that might be contributing to poor symptom control and ongoing asthma management to include patient education. Treatment of severe asthma involves a stepwise approach including phenotype assessment, non-biologic add-on treatment, and biologic add-on treatment with ongoing assessment and asthma management [73].
Asthma is a heterogenous disease with many different phenotypes. A clear distinction has been made between Th2-high and non-Th2 phenotypes. Biologics can be considered for the treatment of severe asthma following an assessment of the patient’s phenotype. A number of biologics have been developed and licensed for use in patients with Th2 asthma (Table 3) [74]. These biologic therapies include agents targeting IgE, IL5, IL5 receptor, and IL4 receptor [75]. However, a lack of comparative studies between different biologic agents would enable doctors to determine which biologic therapy is best suited to their patient [76]. The identification of novel biomarkers could lead to more tailored treatment options for severe asthma patients [76].
| - |
Omalizumab (XOLAIR) |
Mepolizumab (NUCALA) |
Reslizumab (CINQAIR) |
Benralizumab (FASENRA) |
Dupilumab (DUPIXENT) |
|---|---|---|---|---|---|
| FDA approval | Moderate-to-severe persistent asthma in ≥6 years, with a positive skin test or in vitro reactivity to a perennial aeroallergen and symptoms that are inadequately controlled with inhaled corticosteroids | Add-on maintenance treatment for ≥6 years with severe asthma and eosinophilic phenotype | Add-on maintenance treatment for ≥18 years with severe asthma and eosinophilic phenotype | Add-on maintenance treatment for ≥12 years with severe asthma and eosinophilic phenotype | Add-on maintenance treatment for moderate-to-severe asthma ≥12 years with an eosinophilic phenotype or with oral-corticosteroid-dependent asthma |
| Target | IgE | IL5 | IL5 | IL5 receptor | IL4 receptor |
| Route of administration | Subcutaneous | Subcutaneous | Intravenous infusion over 20–50 minutes | Subcutaneous | Subcutaneous |
| Frequency of administration | Every 2–4 weeks | Every 4 weeks | Every 4 weeks | Every 4 weeks for three doses, then every 8 weeks | Every 2 weeks |
| Dose | Based on body weight and pretreatment serum IgE | 40 mg for 6–11 years; 100 mg for ≥12 years | 3 mg/kg body weight | 30 mg | Initial dose 400 mg followed by doses of 200 mg Or initial dose 600 mg followed by doses of 300 mg |
| Benefits | Fewer exacerbations and decreased need for ICS and rescue treatment [77] Improvement in asthma-related quality of life [78] compared with placebo |
Reduced rate of exacerbations and reduced hospitalization or ER visits associated with exacerbations [79] Reduction in dose of OCS [80] compared with placebo |
Reduced rate of asthma exacerbations [81] Enhanced asthma-related quality of life, lung function, and asthma control [82] versus placebo |
Reduced frequency of exacerbations. Improved lung function and symptoms [83] Reduced OCS use [84] compared with placebo |
Reduced number of exacerbations, improved lung function and asthma control [85] Reduction in OCS dose [86] compared with placebo |
| Adverse events as per label | Injection site reactions, viral infections, upper respiratory tract infection, sinusitis, headache, and pharyngitis | Headache, injection site reaction, back pain, and fatigue | Oropharyngeal pain | Headache and pharyngitis | Injection site reactions, conjunctivitis, blepharitis, oral herpes, keratitis, eye pruritus, other herpes simplex virus infection, and dry eye |
| Availability in the Gulf | Yes | Yes | No | Yes | Yes |
3.3. Reviewing Response and when to Refer
3.3.1. Reviewing Response
Patients must examine their asthma approximately 1–3 months after initial treatment commencement and then every 3–12 months [44]. GINA recommends reviewing symptoms, lung function, medication side effects, exacerbations, and patient satisfaction [44]. Symptoms should be assessed using validated questionnaires such as the Asthma Control Test (ACT) or Asthma Control Questionnaire (ACQ) [45].
3.3.2. Adjusting Treatment
Treatment should be stepped up if the current treatment does not control symptoms adequately or stepped down if symptoms have been well controlled for a period of approximately 3 months, in a stepwise manner [44]. When stepping treatment down, patients should be kept on the lowest dose of ICS that controls the symptoms [45].
3.3.3. Referral
Patients who have been hospitalized following an asthma exacerbation, those presenting more than once at hospital due to an exacerbation, or those with difficult-to-treat asthma should be referred to an asthma specialist. Referral may also be required for specialist evaluation if, for example, the diagnosis is not clear [45].
Despite the available guidelines that address the management of severe asthma patients, a large unmet need remains for clear referral routes for these patients through the health system. Patients with severe asthma have the right to receive optimum medical care and the chance to be evaluated by an asthma specialist [87]. Furthermore, many challenges should be addressed at the country level [88]. Patients with severe asthma would benefit from a collaborative effort between primary healthcare centers (PHCs) and asthma specialists to identify them earlier. These collaborative efforts include all possible supporting healthcare professionals to identify appropriate patients with severe asthma who require referral to asthma specialists, provide tools to expedite referral and understand and mitigate challenges to severe asthma patient referrals in primary care.
3.4. Exacerbations
An exacerbation of asthma is the worsening of asthma symptoms and lung function relative to the patient’s usual asthma status: it can place the patient at greater risk of asthma-related death [44]. Patients with severe or life-threatening asthma should be admitted to hospital [45]. A severe asthma exacerbation, in the setting of self-management, is one that requires the use of oral corticosteroids (OCS) or an increase in maintenance dose for >3 days and/or hospitalization or ER visit requiring OCS [89]. Upon presentation with an acute exacerbation, the severity of asthma should be assessed while treatment with oxygen and SABA is initiated; should the patient have life-threatening asthma, they should be treated in an intensive care unit (ICU) [44]. Prompt treatment with SABA, oxygen, and OCS is recommended by GINA, with the addition of ipratropium bromide and SABA nebulizer in the case of severe asthma exacerbations [44]. Where possible, nebulizers may be replaced with a metered dose inhaler (MDI) and spacer to facilitate drug delivery since they are as effective, simple to use, confer fewer side effects, and are less likely to spread respiratory pathogens [90, 91]. Intravenous (IV) magnesium sulfate should also be considered for severe exacerbations not responding to the initial management or in the case of life-threatening asthma [92]. According to GINA, regular (hourly) assessment should be undertaken and admission considered based on symptoms, treatment response, lung function, and history of flare-ups [44]. The BTS/SIGN guidelines recommend admission if the patient has life-threatening asthma or severe asthma that is not improved by the initial phase of treatment [45]. Following the exacerbation, a follow-up appointment should be arranged within 2 to 7 days [44].
4. GULF INSIGHTS, RECOMMENDATIONS, AND KEY PERFORMANCE INDICATORS
4.1. Asthma Diagnosis and Assessment
4.1.1. Asthma Diagnosis
Problems with underdiagnosis have historically been reported, with fear being associated with the word “asthma.” [93, 94] Underdiagnosis has negative implications on asthma-associated morbidity and mortality. More commonly, the lack of presentation of patient symptoms to their GP is the cause of underdiagnosis, rather than a physician’s underdiagnosis. Recently, overdiagnosis has been reported in clinical practice, with a Canadian study reporting that 33.1% of diagnosed asthmatics were not confirmed as having asthma following objective diagnostic testing [95]. Because patients with an existing diagnosis may not be reevaluated or have their diagnosis reconfirmed, the diagnosis will remain in their records. Overdiagnosis can result from a lack of access to appropriate testing, especially in primary healthcare facilities, resulting in a diagnosis being made without confirmation by objective diagnostic testing of variable airflow limitation [96]. Such overdiagnosis has implications for healthcare expenditure as well as risks associated with the use of ICS [97].
In the Gulf region, overdiagnosis is likely to be even higher than in published global data. Many patients presenting with a cough or wheeze are diagnosed with asthma. In Arabic, the term “asthma” incorporates other terms, which may also be a source of overdiagnosis. Data from Kuwait show that 43.1% of physicians have a PEF meter available in their clinic to aid them in making an objective measurement for evidence of a diagnosis. A study in the UAE of asthma patients demonstrated that only 30% of patients had ever had a lung function test; a further 8% of patients had used a PEF meter. Furthermore, obesity is a major problem in the Gulf and can cause symptoms that mimic asthma, leading to an overdiagnosis of asthma without an objective assessment. Obesity causes dyspnea on exertion (although not bronchial hyper-responsiveness) and impairs respiratory muscle function. Furthermore, obese people are more likely than non-obese people to report wheezing [98]. It is therefore easy for obese patients to be misdiagnosed with asthma. Such data highlight a greater need for objective tests or measurements to be used for a more robust asthma diagnosis, to avoid overdiagnosis and overtreatment.
Recommendations:
1. Diagnosis of asthma should involve taking a robust history of symptoms plus an objective measurement or assessment of variable airflow limitation. Normally, more than one visit is needed to make an assessment.
2. Diagnosis of asthma should be confirmed wherever possible; the evidence for the diagnosis of asthma should be documented before starting a controller treatment. It is often more difficult to confirm the diagnosis following treatment.
4.1.2. Asthma Assessment
The assessment of asthma involves determining the level of asthma control using validated questionnaires. Local data concerning asthma control suggest that asthma is not well controlled in the region (Table 4), with the AIM study showing control to be as low as 3% in that set of asthma patients [24]. There is a need for greater knowledge among both physicians and patients about asthma management as well as greater use of controller medications. A Finnish study demonstrated a threefold increase in patients being prescribed maintenance medication between 1987 and 2013; however, there was a decrease in overall asthma costs of 14% [99]. The study demonstrated that the use of maintenance medications can reduce overall costs; such a trend reflects a greater awareness of the disease and more effective treatment of asthma patients within this population [99]. Greater efforts to increase the level of control of patients in the Gulf would benefit the patients as well as reduce the economic burden associated with asthma.
Table 4.
| Countries | Completely or well controlled (%) | Partially controlled or uncontrolled (%) | Assessment | Month / Year | Study |
|---|---|---|---|---|---|
| Kuwait, Saudi Arabia, the UAE, and Russia | 3.0 | 97.0 | GINA | December 2016–February 2017 | AIM [24] |
| 66.0 | 44.0 | Patient-reported | |||
| Jordan, Kuwait, Lebanon, Oman, and the UAE | 52.0 | 48.0 | ACT | January 2007–March 2008 | AIRGNE [23] |
| Algeria, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Qatar, Saudi Arabia, Tunisia, and the UAE |
29.4 | 70.6 | GINA | June 2014–December 2015 | ESMAA [100] |
| 51.4 | 48.5 | ACT (patient-reported) | |||
| Qatar | 41.0 | 59.0 | GINA | May 2011–March 2014 | QASMA [101] |
4.1.2.1. Asthma and Comorbidities
Comorbidities associated with asthma should be assessed at diagnosis. Obesity has been associated with increased incidence of asthma as well as poor asthma control [102]. Obesity rates are high in the Gulf region, ranging in 2016 from 27.0% in Oman to 40.2% in Kuwait [103]. Challenges relating to obesity in the Gulf include the consumption of fast food as well as physical inactivity and, despite the implementation of health promotion policies, barriers to dealing with obesity still remain [104]. There is also some evidence of high prevalence of obstructive sleep apnea (OSA) in asthma patients, which is also strongly associated with obesity [105]. OSA is highly prevalent in the Gulf, with 20.3% of patients older than 14 years presenting with symptoms of OSA in the UAE [106]. Gastroesophageal reflux disease (GERD) and asthma are often found together; however, the mechanism of association remains unclear. Theories include the aspiration of gastric content resulting in asthma as well as acidification of the lower esophagus resulting in smooth muscle contraction of the bronchus [107]. The ESMAA study reported a 24% incidence of GERD regionally in asthma patients [100]. Globally, however, studies have shown a much higher prevalence of GERD in asthma patients, of 71% [108]. Allergic rhinitis is an other asthma-related comorbidity. Allergic rhinitis is common in the Gulf, with a prevalence of 30.5% in Qatar and 36% in Al-Ain, UAE [31, 109]. Asthma and allergic rhinitis were found to be highly associated, with 59.9% of school children with asthma also having allergic rhinitis [31].
Smoking regularly as well as exposure to smoking in utero has been associated with increased asthma risk in adolescents [110]. There is a high prevalence of current tobacco smoking in the Gulf states, ranging in 2019 from 7.8% in Oman to 19.3% in Kuwait [111]. Furthermore, water pipe smoking is also relatively common in the Middle East, with a 3.5% consumption rate [112], which is also associated with an increased risk of asthma and asthma exacerbations in adults [113] as well as in childhood following in utero exposure [114]. Despite the implementation of smoke-free policies in the Gulf, some policies are minimal or weak according to the WHO and their compliance may be low [111]. It is clear that, in the Gulf region, asthma management faces several barriers associated with a higher and rising prevalence of these comorbidities. Further health promotion policies are required to tackle and address the burden of these comorbid conditions, which will in turn help to reduce the burden of asthma in the region.
Recommendations:
1. Comorbidities, such as obesity, GERD, allergic rhinitis, obstructive sleep apnea, depression/anxiety, and eczema should be assessed and addressed at diagnosis and at further review.
2. Smoking status of asthmatic patients should be determined at diagnosis and at further review. Steps should be taken to promote and help patients with smoking cessation.
4.1.3. Asthma-Focused Healthcare
Asthma clinics have been shown to greatly benefit asthma patients by reducing the morbidity associated with the condition [115]. Such clinics can educate the patient regarding their condition as well as review the inhaler techniques and undertaking spirometry, PEF measurements, and reversibility testing. Asthma clinics have been established in Oman since 2010 and have benefited the management of asthma in the country with greater documentation of case signs and symptoms, history, triggering factors, and comorbidities as well as increased prescription of ICS and SABA [116]. In 2018, there were 152 asthma clinics in the Ministry of Health Primary Healthcare Centers Oman, covering 63% of all PHCs (unpublished registry data). In 2017, a reported 11,750 patients were registered at these asthma clinics across Oman (unpublished registry data). These asthma clinics reported a reduction in emergency visits (25.4% at baseline visit vs. 15.0% at last follow-up visit) and hospitalizations (7.9% at baseline visit vs. 3.1% at last follow-up visit) during the past 12 months in registered patients (unpublished registry data). Furthermore, there was an increase in patients reporting good asthma control according to ACT (32.0% at baseline visit vs. 56.0% at the last follow-up visit) during the past 12 months in registered patients (unpublished registry data).
Asthma clinics have been established in Kuwait since early 2000. Patients attending asthma clinics in Kuwait demonstrated greater knowledge about their condition and potential triggers, had greater use of steroid medication and less use of bronchodilator medication, had fewer attacks, and had a greater prevalence of correct inhaler techniques compared with asthma patients attending non-asthma clinics [117].
Asthma educators have been instrumental in many countries in providing patient education at primary care centers, which has been shown to increase patient adherence to medication, increase asthma literacy, and facilitate greater self-management [118, 119]. A Cochrane review has demonstrated that educational outreach visits have small but consistent effects on prescribing [120]. Furthermore, physician education is more likely to be successful if it includes a mixture of interactive and didactic education rather than interactive or didactic alone [121]. There is a huge unmet need for asthma educators within the Gulf healthcare systems.
Recommendations:
1. There is a need for well-established primary healthcare systems to establish asthma clinics with open access for patients.
2. In primary health care, people with asthma should be reviewed regularly by a doctor with appropriate training in asthma management.
3. Asthma educators should be utilized for the optimal management of asthma.
4. Training for primary care clinicians should include educational outreach visits using multifaceted programs that include consultation training and goal setting.
4.1.4. Key Performance Indicators
1 Percentage of asthma patients diagnosed with a confirmed variability of expiratory airflow limitation and with a clear rationale for diagnosis documented in their medical records.
(Denominator: Total number of asthma patients)
2 Percentage of asthma patients who have had comorbidities identified, including allergic rhinitis, obesity, obstructive sleep apnea, depression/anxiety, and/or eczema.
(Denominator: Total number of asthma patients)
3 Percentage of asthma patients who have been reviewed in primary care asthma clinics by a doctor with appropriate training in asthma management.
(Denominator: Total number of asthma patients)
4.2. Asthma Management
4.2.1. Treatment
The recommended stepwise treatment approach in adults is shown in Fig. (2). Low-dose ICS-containing treatment is now recommended for all adults and adolescents with asthma to reduce the risk of serious exacerbations [44]. For mild asthma, budesonide/formoterol as needed is recommended, whereas for moderate asthma, low-dose budesonide/formoterol or beclomethasone/formoterol are recommended as maintenance and reliever [44]. This represents a population-level recommendation, which represents the best treatment option for most of the asthma population; individual circumstances should, however, always be considered [44]. There is increasing evidence for adverse clinical outcomes associated with the use of SABA for the treatment of asthma. Use of three or more cannisters annually is associated with a higher risk of asthma exacerbations. Furthermore, increased mortality is also associated with greater use of beta-agonists [122, 123]. Use of two or more cannisters per month (or ≥12 cannisters per year) is associated with a higher risk of asthma-related death [124]. Therefore, for safety reasons, GINA no longer recommends SABA-only treatment for step 1 of the stepwise approach.
In the Asthma Insights and Reality (AIR) study, conducted in the UAE, less than one-third (31%) of patients were aware of ICS with 5.5% of patients using them. Furthermore, more than two-thirds (67%) were currently using SABA for the treatment of their asthma, with 57% of patients believing SABA to be the most effective treatment for asthma control. These data provide evidence for patients’ lack of knowledge concerning their condition and appropriate treatment as well as poor adherence to guidelines [125]. There is a need for greater education of physicians and patients regarding treatment in light of the recent updates to the GINA guidelines.
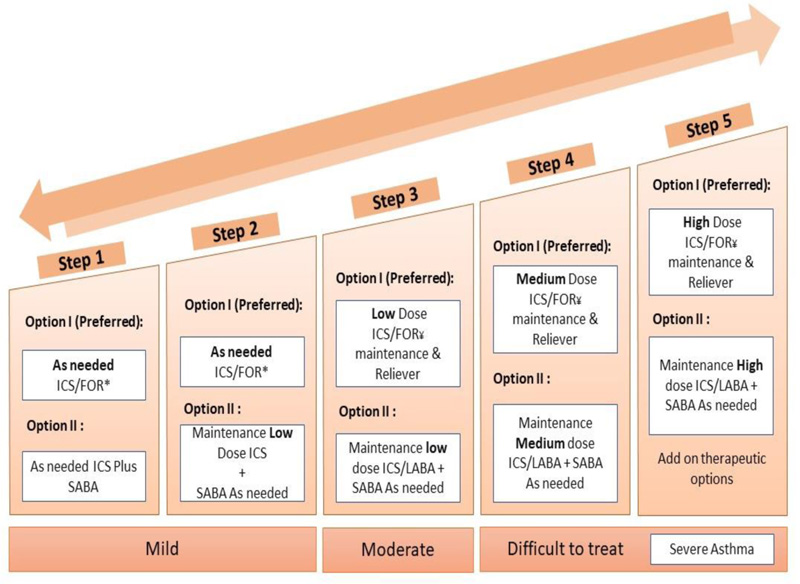
GINA recommends the use of as-needed low-dose ICS/formoterol as the preferred controller in patients with mild asthma. Alternatively, low-dose ICS can be taken each time SABA is used [44]. However, the two are often taken in separate inhalers and the advantages of ICS treatment are often limited due to a lack of adherence in mild asthma cases [126]. ICS/SABA treatment combinations, which are being investigated, may be a useful alternative and mitigate the problem of adherence in such cases.
Asthma action plans have been demonstrated to result in early self-management of exacerbations of asthma as well as reduced rates of hospitalization, ICU admission, ER visits, and sick days [127]. Furthermore, adult asthma patients with asthma action plans have been demonstrated to have a greater number of adherence days to their medication and greater avoidance of their asthma triggers compared with patients without asthma action plans [127]. Similar benefits have been observed in children, with reduced ER visits, outpatient visits, and school absences [128]. Symptom-based asthma action plans appear to be more beneficial in children than peak flow asthma action plans [129]. A lack of asthma action plans provided by physicians has been reported in the region [23].
Validated patient questionnaires assessing asthma control include the ACT and the ACQ [56, 130, 131].
Recommendations:
1. SABA should not be used alone for the treatment of asthma.
2. As-needed low-dose ICS/formoterol should be used in mild asthma cases.
3. There is a need to build on the evidence base of combination ICS/SABA use as a second option, in cases where ICS/formoterol is not available and to expedite the availability of ICS/SABA combinations in the market. This approach may be a useful alternative to mitigate the problem of adherence with two separate devices.
4. Treatment optimization using the ICS-formoterol maintenance and reliever approach should be considered in moderate to severe cases.
5. There is a need to educate and upskill doctors, allied health workers, and patients about the latest changes in the GINA guidelines.
6. There is a need for better communication between physicians at primary and secondary or tertiary healthcare centers to ensure continuity of care, including access to records and documentation of a clear management plan.
7. All asthmatics should be provided with a personalized written asthma action plan. This should be reviewed at every visit or as needed.
8. Asthma control should be assessed based on an objective asthma control questionnaire, e.g., ACT or ACQ.
4.2.2. Key Performance Indicators
1 Percentage of asthma patients who were initiated on ICS or ICS/LABA combination at diagnosis.
(Denominator: Total number of asthma patients)
2 Percentage of asthma patients who have had a personalized written asthma management plan initiated at diagnosis.
(Denominator: Total number of asthma patients)
3 Percentage of asthma patients who have received asthma education from a certified asthma educator at diagnosis.
(Denominator: Total number of asthma patients)
4 Percentage of asthmatic smokers who have received smoking cessation advice in the past 12 months.
(Denominator: Total number of asthma patients followed-up for at least 12 months)
5 Percentage of asthma patients with asthma severity documented according to the GINA 2020 guidelines classification.
(Denominator: Total number of asthma patients)
6 Percentage of primary healthcare facilities with available prophylactic treatment for asthma (Denominator: Total number of primary healthcare facilities in a region/district/country) *.
* Key performance indicators have been developed and implemented in Oman Primary Care by the Non-Communicable Diseases Department
4.3. DIFFICULT-TO-TREAT AND SEVERE ASTHMA MANAGEMENT
4.3.1. Treatment
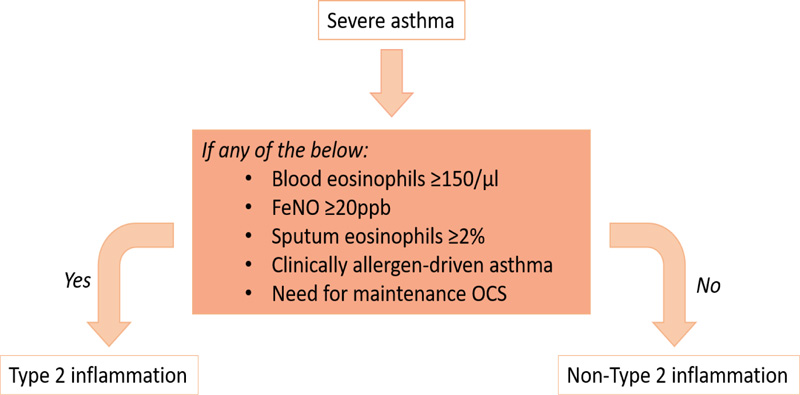
Management of difficult-to-treat and severe asthma, as detailed in Fig. (3), involves the optimization of treatment including add-on therapy and considering a strategy of maintenance and reliever treatment using ICS-formoterol. As with all levels of asthma severity, asthma education as well as risk factors and comorbidity assessment and treatment is key to managing the condition. Assessment of the severe asthma phenotype involves assessment of markers for type 2 inflammation, including FeNO and serum and sputum eosinophils (Fig. 4). Such assessment tools are not available at all treatment centers across the region and therefore, referral to a center of excellence may be necessary to confirm the phenotype in severe asthmatics.
In the Gulf region, omalizumab, mepolizumab, benralizumab, and dupilumab are currently available. However, there is a lack of published data describing the use of these biologics in the region. Long-term omalizumab treatment of patients in Kuwait has been shown to be effective, with reduced frequency of exacerbations, fewer hospitalizations, and ER visits, reduced OCS and ICS/LABA use, increased asthma control, and improvements in lung function [132, 133].
Recommendation:
1. There should be additional support for the availability of tools that can help physicians to phenotype severe asthmatics, such as FeNO, allergy testing, etc.

4.3.2. Key Performance Indicators
1 Percentage of severe asthmatic patients on biologics for whom decision to initiate biologic treatment was taken through a regional or national multidisciplinary team meeting at a severe asthma center.
(Denominator: Total number of severe asthma patients on biologic therapy)
2 Percentage of severe asthma patients taking biologics who meet the GINA criteria.
(Denominator: Total number of severe asthma patients on biologic therapy)
4.4. Reviewing Response and when to Refer
4.4.1. Reviewing a Response and Adjusting Treatment
A key area of assessment when reviewing a patient’s response to treatment is ensuring their adherence to their asthma medication. Data from the UAE have shown that although current ICS use is 5.5%, quick relief treatment is 47.5%, giving a very low ratio of ICS to SABA use (0.12%), which has been suggested to reflect poor control in this set of patients. There is therefore a need for greater efforts to increase the awareness and knowledge of asthma patients regarding their treatment and the importance of compliance to effectively manage their asthma. A study involving 218 asthma patients in Oman demonstrated that poor patient adherence to medication (40.8%) and poor inhaler technique (18.3%) were factors contributing to poor asthma control (57.8%) [134]. Assessing the patient for adequate inhaler technique is another important aspect of asthma review. A study of 450 asthma patients visiting emergency departments in Saudi Arabia demonstrated that 45% of patients had an incorrect inhaler technique and 40% did not follow up consistently with a physician, leading to poor asthma control and frequent visits to the emergency department. Patient management requires regular assessment of patients and their attendance at follow-up appointments after their initial diagnosis. Data from the UAE show that few patients (17%) had scheduled follow-up appointments. This highlights the need for greater education of patients to ensure ongoing follow-up and review of their condition are achieved. The creation and/or review of a personalized written asthma action plan is also recommended at each asthma review. Data from the UAE show that only 15% of patients had written action plans for their asthma. Finally, there is a need for greater adherence to published guidance by physicians.
Recommendations:
1. It is important to check patients’ adherence to their treatment at all follow-up appointments.
2. A review of inhaler techniques and objective assessment of asthma control must be undertaken at all follow-up appointments.
3. Personalized asthma action plans should be reviewed or provided if not already in place.
4. Stepping treatment up (when symptoms remain uncontrolled) or down (when asthma is controlled ≥3 months) are to be considered at each visit.
4.4.2. Referral
The following referral algorithm, ReferID, is a simple logic-based tool driven by four “red flag” questions designed to help healthcare providers identify patients with a confirmed diagnosis and who are already on regular prophylactic medication who may benefit from referral to a specialist. It was designed based on GINA guidelines by five international experts in asthma and funded by AstraZeneca [135].
1. Has the patient used two or more courses of systemic corticosteroids (SCS) and/or is using maintenance SCS therapy over the past 12 months?
2. Has the patient had two or more emergency attendances/unscheduled visits due to asthma over the past 12 months?
3. Has the patient ever been intubated or admitted to an ICU or high dependency unit due to their asthma?
4. How many SABA inhalers has the patient used over the past 12 months?
Review by an asthma specialist is recommended if “Yes” is answered to questions 1, 2, or 3 or if three or more SABA inhalers were used by the patient in the past 12 months.
Recommendations:
5. Referral of a patient should be based on the above referral algorithm.
4.4.3. Key Performance Indicators
1 Percentage of asthma patients referred to an asthma specialist based on exacerbations, symptom control, adherence, inhaler technique, and risk factor assessment.
(Denominator: Total number of asthma patients referred to an asthma specialist)
2 Percentage of asthma patients who have a personalized written asthma action plan which was reviewed at their last follow-up appointment.
(Denominator: Total number of asthma patients with a personalized asthma action plan)
3 Percentage of asthma patients with confirmed severe or difficult-to-treat asthma who have been referred to a center of excellence (a facility providing high-quality best practices, leadership, training, and support in asthma).
(Denominator: Total number of confirmed severe or difficult-to-treat asthma patients).
4 Percentage of asthma patients who have had their inhaler technique and adherence checked at their last visit or within the past 6 months.
(Denominator: Total number of asthma patients)
5 Percentage of asthma patients who have had appropriate lung function testing recorded at their most recent structured visit or within the past year.
(Denominator: Total number of asthma patients)
6 Percentage of asthma patients who have experienced exacerbations or unscheduled visits to healthcare facilities within the past 12 months.
(Denominator: Total number of asthma patients followed-up for at least one year)
7 Percentage of asthma patients who have a documented the standardized asthma control questionnaire recorded at their last visit.
(Denominator: Total number of asthma patients)
8 Percentage of at-risk asthma patients (defined as >1 severe exacerbation per year, ever intubated, those with learning disabilities, those under using ICS or showing poor concordance with treatment) who have a regular review appointment arranged.
9 Percentage of asthma patients following up for a year in a PHC asthma clinic and with adequate control (ACT ≥20) of their asthma 1 year after initiation of management in the clinic*.
(Denominator: Number of asthma patients who have been followed-up for at least one year)
10 Percentage of asthma patients who have been on follow-up for at least 1 year and have been hospitalized during the past 12 months*.
(Denominator: Number of asthma patients who have been followed-up for at least one year)
11 Percentage of asthma patients who have been on follow-up for at least 1 year and have had emergency visits during the past 12 months*.
(Denominator: Number of asthma patients who have been followed-up for at least one year)
12 Percentage of people who have died from asthma (where asthma is the underlying cause of death or listed as an associated cause of death)*.
(Denominator: Estimated resident population)
* KPIs have been developed and implemented in Oman Primary Care by the Non-Communicable Diseases Department.
4.5. Exacerbations
Exacerbations pose a significant burden on the healthcare system. In asthma patients within the region, rates of hospitalization due to asthma in the past year ranged from 4.0% in the UAE to 27.4% in Kuwait, while rates of ER visits due to asthma in the past year ranged from 27.5% in the UAE to 89.1% in Kuwait [23]. Such high rates are thought to result from the lack of use of controller medication in the region. There is a need for increased awareness of ICS-containing medications for the treatment of asthma within the region, guided by the recent updates to the GINA guidelines [44]. There is increasing evidence for the role of the maintenance and reliever approach (budesonide-formoterol and beclomethasone-formoterol) in the management of asthma and reducing the number of exacerbations via adjustable doses [136]. Therefore, this approach may be preferable for asthma patients with frequent exacerbations.
Recommendations:
1. The number of exacerbations and hospitalizations as well as unscheduled visits to healthcare facilities or practitioners must be tracked.
2. Patients should be discharged with a personalized written asthma action plan.
3. Patients should be discharged with a follow-up in primary care within 2–5 days and a follow-up in specialist clinics within a month of discharge, if needed.
4. Patients should be offered smoking cessation advice, if needed.
5. Identify triggers of the acute attack, if possible, and discuss future management plans for exposure.
6. Clinical assessment of asthma exacerbation severity, including respiration rate, oxygen saturation, PEF, heart rate, and use of accessory muscles, should be undertaken by triage nurses as early as possible upon presentation to the ER.
7. OCS to be administered within 1 hour of arrival in the ER; or IV corticosteroids for dyspneic or vomiting patients or those requiring non-invasive ventilation or intubation.
8. Patients must not be discharged from the ER on SABA alone; ICS must be prescribed alongside SABA.
9. Following ER admission, physicians must know the correct disposal of their patients on discharge, inpatient, or ICU, have a discharge plan, and provide the patient with a written asthma action plan.
10. Follow-up of patients after discharge from the ER is essential to support patients in continuing treatment and symptom control and to assess lung function.
4.5.1. Key Performance Indicators
1 Percentage of asthma patients who have been hospitalized for asthma exacerbation and were discharged with a follow-up review in either primary care or specialist clinics within 2 to 7 days.
(Denominator: Total number of asthma patients who have been hospitalized for an asthma exacerbation)
2 Percentage of asthma patients who have been hospitalized for an asthma exacerbation and were discharged with a written asthma action plan or a review of an already existing asthma action plan.
(Denominator: Total number of asthma patients who have been hospitalized for an asthma exacerbation)
3 Percentage of asthma patients who have been hospitalized for an asthma exacerbation and discharged with a review of their treatment plan and their inhaler techniques.
(Denominator: Total number of asthma patients who have been hospitalized for an asthma exacerbation)
4 Percentage of asthma patients who have been hospitalized for an asthma exacerbation and were seen by an asthma educator during their hospital stay.
(Denominator: Total number of asthma patients who have been hospitalized for an asthma exacerbation)
5 Percentage of asthma patients who have been hospitalized for an asthma exacerbation and were discharged with controller medication.
(Denominator: Total number of asthma patients who have been hospitalized for an asthma exacerbation)
4.6. Further Insights, Goals, and Recommendations
4.6.1. Asthma Registries
Asthma registries can provide valuable information including patient demographics, asthma phenotypes, treatment utilization patterns, and medication side effects. Such registries have been implemented globally as well as in individual country settings such as the UK [137], Italy [19], Belgium [138], and Portugal [139] for severe asthma. The benefits of such registries include the large volume of data, which can be of benefit to researchers; however, data must be validated and checked regularly to ensure it is of high quality. Registries only provide very specific information relating to certain regions or countries; it is therefore important for sites or countries within the Gulf to create their own registry of cases to gain insight into the specific characteristics and phenotypes of cases in the region.
4.6.2. Digital Devices
Previously discussed adherence issues related to asthma treatment can often be compounded by complicated treatment regimens involving regular and as-needed treatment as well as multiple inhaler types [140]. Digital devices have been shown to enable the patient to improve adherence to their treatment, including the use of “smart” inhalers that can provide reminders to the patient [141, 142]. Furthermore, smartphone applications have been shown to enable self-tracking of symptoms, control, and adherence as well as provide data concerning factors that might trigger asthma symptoms such as weather, air quality, and pollen [143]. Given the digital age and high levels of device use, it may be possible to encourage adherence in patients in the Gulf using digital devices.
4.6.3. Electronic Templates
Electronic templates for clinical appointments can be of great benefit by enabling physicians to provide structured management of patients, facilitate the provision of summary data, and enable communication and continuation of care among doctors treating the same patient. However, such templates may also be considered restrictive and provide little flexibility to physicians in the management of complex patients. Templates may alternatively serve as a guide to remind physicians rather than a restrictive tool [144]. The use of electronic templates may be of some benefit in the Gulf to guide physicians in the management of asthma patients and to provide reminders to collect specific data and parameters set out by the guidelines.
Recommendations:
1. Healthcare facilities should have a registry to facilitate the auditing and documentation of asthma and especially severe asthma cases.
2. Digital devices as well as monitoring and communication templates should be implemented for better patient control.
CONCLUSION
While it is recommended that the GINA guidelines should be followed wherever possible for the management of asthma, key opinion leaders in the Gulf region have presented additional recommendations based on regional challenges and insights. There is a need for better diagnosis using objective testing, increased efforts in tackling the burden of comorbidities in the region, such as obesity, smoking, and GERD, and greater provision of the necessary tools for phenotyping severe asthma. Furthermore, there is a need for better education of physicians regarding asthma treatment in light of the recent updates to GINA, including the shift away from SABA-only treatment and the importance of ICS-containing controller medication. Regionally, there is also a need for specialist asthma clinics and asthma educators, which would serve to educate physicians and their patients as well as to improve the management of patients. Finally, the use of asthma registries, digital devices, and electronic templates would benefit the management of asthma patients in the region.
LIST OF ABBREVIATIONS
| KOLs | = Key Opinion Leaders |
| BDR | = Bronchodilator Response |
| PEF | = Peak Expiratory Flow |
| MTC | = Metcholine Testing Challenge |
| GINA | = Global Initiative for Asthma |
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
Support for Expert Panel Steering Committee meeting logistics was provided by AstraZeneca. Funding for medical writing assistance was provided by AstraZeneca. The review is a fully independent effort with no external involvement other than external medical writing support.
CONFLICT OF INTEREST
NB has received speaker honoraria and sponsorship to attend conferences from AstraZeneca, Boehringer Ingelheim, GSK, Pfizer, and Novartis. All other authors report no conflict of interest.
ACKNOWLEDGEMENTS
The authors thank Abigail Holland of Connect Communications for medical writing assistance.


