The Association of Latitude and Altitude with COVID-19 Symptoms: A VIRUS: COVID-19 Registry Analysis
Abstract
Background:
Better delineation of COVID-19 presentations in different climatological conditions might assist with prompt diagnosis and isolation of patients.
Objectives:
To study the association of latitude and altitude with COVID-19 symptomatology.
Methods:
This observational cohort study included 12267 adult COVID-19 patients hospitalized between 03/2020 and 01/2021 at 181 hospitals in 24 countries within the SCCM Discovery VIRUS: COVID-19 Registry. The outcome was symptoms at admission, categorized as respiratory, gastrointestinal, neurological, mucocutaneous, cardiovascular, and constitutional. Other symptoms were grouped as atypical. Multivariable regression modeling was performed, adjusting for baseline characteristics. Models were fitted using generalized estimating equations to account for the clustering.
Results:
The median age was 62 years, with 57% males. The median age and percentage of patients with comorbidities increased with higher latitude. Conversely, patients with comorbidities decreased with elevated altitudes. The most common symptoms were respiratory (80%), followed by constitutional (75%). Presentation with respiratory symptoms was not associated with the location. After adjustment, at lower latitudes (<30º), patients presented less commonly with gastrointestinal symptoms (p<.001, odds ratios for 15º, 25º, and 30º: 0.32, 0.81, and 0.98, respectively). Atypical symptoms were present in 21% of the patients and showed an association with altitude (p=.026, odds ratios for 75, 125, 400, and 600 meters above sea level: 0.44, 0.60, 0.84, and 0.77, respectively).
Conclusions:
We observed geographic variability in symptoms of COVID-19 patients. Respiratory symptoms were most common but were not associated with the location. Gastrointestinal symptoms were less frequent in lower latitudes. Atypical symptoms were associated with higher altitude.
1. INTRODUCTION
After being identified in Wuhan, China, at the end of 2019, Coronavirus disease 2019 (COVID-19) rapidly disseminated worldwide and affected millions [1, 2]. The global vaccination process has begun [3]; however, isolation of the infected patients still represents one of the most crucial measures in preventing the morbidity and mortality of COVID-19 [4-6]. Prompt identification of potential cases would make prevention more efficient [7]. To that end, gaining more insight into disease symptomatology plays a pivotal role, particularly in limited-resource settings. Furthermore, recognizing the prevalence of relatively uncommon presentations in different settings helps determine the extent of the pandemic [8].
Manifestations like cutaneous symptoms and anosmia were later recognized along the pandemic’s timeline of spread worldwide [9, 10]. Accordingly, there were multiple updates to the list of signs recommended to be surveyed for COVID-19 [11]. Nevertheless, it is unclear whether there is a connection between more recently recognized symptoms and the environmental characteristics of the places that encountered the pathogen later in the process. A link between climatological factors and case and fatality rates of COVID-19 has been suggested, attributed to elements like ultraviolet B-light or physiological and habitual characteristics of populations [12-17]. Still, it has not been elucidated whether the abovementioned factors also are associated with signs and symptoms at the disease presentation. Recent studies confirmed that symptoms vary according to age and sex [18, 19]. Also, heterogeneity between different countries was suggested. The difference was attributed to local criteria for testing and sociocultural variations between populations [19]. However, to the best of our knowledge, the effect of latitude and altitude on disease manifestations has not been reported to date.
Viral Infection and Respiratory Illness Universal Study (VIRUS): The COVID-19 registry is a global project with collaborators from 287 sites in 40 US states and 23 other countries [20]. As of this date, it is the second most extensive database of COVID-19 patients. Therefore, the VIRUS: COVID-19 registry provides a good ground for studies that evaluate and compare different aspects of the patients and the disease [21-25]. We aimed to investigate whether climatological and geographical factors, specifically altitude and latitude, impact the signs and symptoms of the VIRUS: COVID-19 registry subjects at the time of admission.
2. METHODS
This analysis was conducted as an ancillary project on data collected within the scope of the VIRUS: COVID-19 registry global study. The institutional review board at Mayo Clinic evaluated the project and approved it as exempt (IRB:20-002610).
The clinical trials database registration number for the registry is NCT04323787.
2.1. Study Population
All subjects hospitalized with a COVID-19-associated indication (laboratory-confirmed or clinically diagnosed infection) at participating institutions were eligible for inclusion in the VIRUS: COVID-19 registry [20]. De-identified data were collected through Research Electronic Data Capture software (REDCap, version 8.11.11, Vanderbilt University, Nashville, Tennessee) and stored in a central database hosted by Mayo Clinic [26]. Although enrolled in the VIRUS: COVID-19 registry, pediatric patients (<18 years old) were not included in this project. All adult subjects admitted between March 2020 and January 2021 were screened for inclusion. Since the option “None” was added to the symptoms data variable later in the study, patients who had no symptoms at the time of admission were excluded from the analyses.
2.2. Data Regarding Patient Location
Due to the de-identified database, the patients' residential locations were unavailable at the time of disease presentation. Instead, the locations of the participating institutions were used as a surrogate to determine the geographical variables. Latitude and altitude information was retrieved from the Google Earth software [27]. Based on their locations, subjects were grouped according to the elevation above sea level and the distance from the equator, regardless of the hemisphere of location [28, 29].
2.3. The Outcome of Interest
The outcome of the study consisted of the signs and symptoms at the time of hospital admission. The symptoms listed in the data collection forms corresponded to symptoms listed in major references, such as the Centers for Disease Control and Prevention and World Health Organization (Supplementary Table 1). For this study, they were categorized according to respiratory, gastrointestinal, neurological, mucocutaneous, and cardiovascular systems. Constitutional symptoms were considered a separate category. For any other symptom not listed specifically in the data collection forms and thus listed as free texts, we used keywords to link them to proper categories. The remaining symptoms, which could not be classified in any pre-specified categories, such as dysuria, insomnia, etc., were included under a separate group as atypical symptoms.
2.4. Statistical Analyses
Institutions that recorded signs and symptoms for ≥75% of their subjects at the time of admission were included in this analysis, while others were considered not fully participating. Continuous data were summarized as the median and interquartile range (IQR), and categorical variables were reported as numbers and percentages.
Unadjusted and multivariable-adjusted logistic regression assessed the association between latitude and the presence of respiratory signs and symptoms at admission. Models were fitted using generalized estimating equations for accounting for the clustering of patients within sites using an exchangeable working correlation. The functional form of continuous latitude was assessed using a Wald test to compare the fit of a restricted cubic spline to the linear functional form. Results are summarized graphically using the restricted cubic spline fit when results suggest a non-linear functional form; the linear relationship is described. Models were adjusted for age, sex, and comorbidities. Comorbidities present in ≥15% of the study sample were included in the calculations, which were hypertension, diabetes, coronary artery disease, dyslipidemia, and obesity. Similar regression models were fitted for gastrointestinal, neurological, mucocutaneous, cardiovascular, constitutional, and atypical symptoms. Analyses were repeated for altitude for each outcome. Odds ratios and 95% confidence intervals were calculated per 10 degrees of latitude and 250-meters of altitude, compared with the reference points of 45° latitude and 175 meters above sea level (m.a.s.l.) altitude.
The study had no missing data as age, sex, and site latitude/altitude was complete. Comorbidities were a ‘check all that apply’ field, so unchecked is assumed not present. Similarly, signs and symptoms outcomes were a ‘check all that apply and unchecked is assumed not present. As described previously, sites with <75% of patients with at least one sign/symptom were excluded as non-participating since they are unlikely to reflect a plausible distribution of signs/symptoms. Statistical significance was specified as p<.05, without adjustment for multiplicity related to testing seven outcomes or testing both altitude and latitude in regression models.
3. RESULTS AND DISCUSSION
After excluding pediatric and asymptomatic patients, 42987 out of 49201 subjects were eligible for this study. And after exclusion of the patients coming from centers with <75% recorded signs and symptoms data, 12267 patients were included in the analysis (Fig. 1). The patients were from 181 hospitals located in 24 countries (Supplementary Table 2). The median age was 62 (49-73) years, and 57% of the patients were male. As for race and ethnicity, 49% were White, followed by 22% Black/African Americans, and 66% were non-Hispanic. Of the study population, 86% had at least one comorbidity, the most common being hypertension (54%), followed by diabetes mellitus (35%). When baseline data were evaluated within latitude and altitude groups, the median age was higher further from the Equator (52 versus 72 years at latitudes of 0-15º and 45-60º, respectively). Similarly, the percentage of female patients (34% and 46% at latitudes of 0-15º and 45-60º, respectively) and patients with comorbidities (53% and 92% at latitudes of 0-15º and 45-60º, respectively) differed based on latitude. On the other hand, the ratio of females (43% and 34% at altitudes of <500 m.a.s.l. and >1000 m.a.s.l., respectively) and patients with any comorbidity (87% and 69% at altitudes of <500 m.a.s.l. and >1000 m.a.s.l., respectively) was lower at elevated altitudes. The distribution of baseline characteristics to different latitude and altitude groups is outlined in Table 1.
| Variables | Total (n=12267) | Latitude | Altitude | |||||
| 0-15° (n=666) | 16-30° (n=2306) | 31-45° (n=8900) | 46-60° (n=395) | <500 m.a.s.l. (n=11133) | 500 – 1000 m.a.s.l. (n=907) | >1000 m.a.s.l. (n=227) | ||
| Age, median, IQR | 62 (49-73) | 52 (38-64) | 59 (46-70) | 63 (50-74) | 72 (59-83) | 62 (50-74) | 57 (45-68) | 60 (49-72) |
| Gender | ||||||||
| Female | 5222 (43%) | 225 (34%) | 929 (40%) | 3885 (44%) | 183 (46%) | 4834 (43%) | 310 (34%) | 78 (34%) |
| Male | 7036 (57%) | 441 (66%) | 1376 (60%) | 5007 (56%) | 212 (54%) | 6290 (57%) | 597 (66%) | 149 (66%) |
| Race | ||||||||
| White | 6038 (49%) | 3 (0%) | 534 (23%) | 5213 (59%) | 288 (73%) | 5755 (52%) | 228 (26%) | 55 (24%) |
| Black/African American | 2673 (22%) | 74 (11%) | 510 (22%) | 2056 (23%) | 33 (8%) | 2652 (24%) | 17 (2%) | 4 (2%) |
| South Asian | 1109 (9%) | 367 (55%) | 689 (30%) | 50 (1%) | 3 (1%) | 887 (8%) | 211 (24%) | 11 (5%) |
| Mixed race | 560 (5%) | 194 (29%) | 142 (6%) | 223 (3%) | 1 (0%) | 297 (3%) | 129 (14%) | 134 (59%) |
| Others | 1887 (15%) | 28 (4%) | 431 (19%) | 1358 (15%) | 70 (17%) | 1542 (14%) | 322 (36%) | 23 (10%) |
| Ethnicity | ||||||||
| Hispanic | 1977 (16%) | 127 (19%) | 417 (18%) | 1427 (16%) | 6 (2%) | 1696 (15%) | 205 (23%) | 76 (33%) |
| Non-Hispanic | 8091 (66%) | 355 (54%) | 1424 (62%) | 6076 (69%) | 236 (60%) | 7634 (69%) | 397 (44%) | 60 (26%) |
| Unknown or others | 2090 (17%) | 181 (27%) | 447 (19%) | 1310 (14%) | 152 (39%) | 1803 (16%) | 305 (34%) | 91 (40%) |
| Comorbidities (any) | 10532 (86%) | 355 (53%) | 1839 (80%) | 7975 (90%) | 363 (92%) | 9722 (87%) | 653 (72%) | 157 (69%) |
| Hypertension | 6625 (54%) | 224 (34%) | 1201 (52%) | 4959 (56%) | 241 (61%) | 6177 (55%) | 362 (40%) | 86 (38%) |
| Diabetes | 4273 (35%) | 166 (25%) | 853 (37%) | 3127 (35%) | 127 (32%) | 3919 (35%) | 298 (33%) | 56 (25%) |
| Coronary artery disease | 2982 (24%) | 39 (6%) | 393 (17%) | 2471 (28%) | 79 (20%) | 2856 (26%) | 104 (11%) | 22 (10%) |
| Dyslipidemia | 2381 (19%) | 7 (1%) | 365 (16%) | 1978 (22%) | 31 (8%) | 2271 (20%) | 98 (11%) | 12(5%) |
| Obesity | 2314 (19%) | 37 (6%) | 425 (18%) | 1790 (20%) | 62 (16%) | 2153 (19%) | 130 (14%) | 31 (14%) |
| Chronic kidney disease | 1727 (14%) | 10 (2%) | 265 (11%) | 1376 (15%) | 76 (19%) | 1651 (15%) | 65 (7%) | 11 (5%) |
| Neurological disorders | 1305 (11%) | 1 (0%) | 166 (7%) | 1060 (12%) | 78 (20%) | 1255 (11%) | 42 (5%) | 8 (4%) |
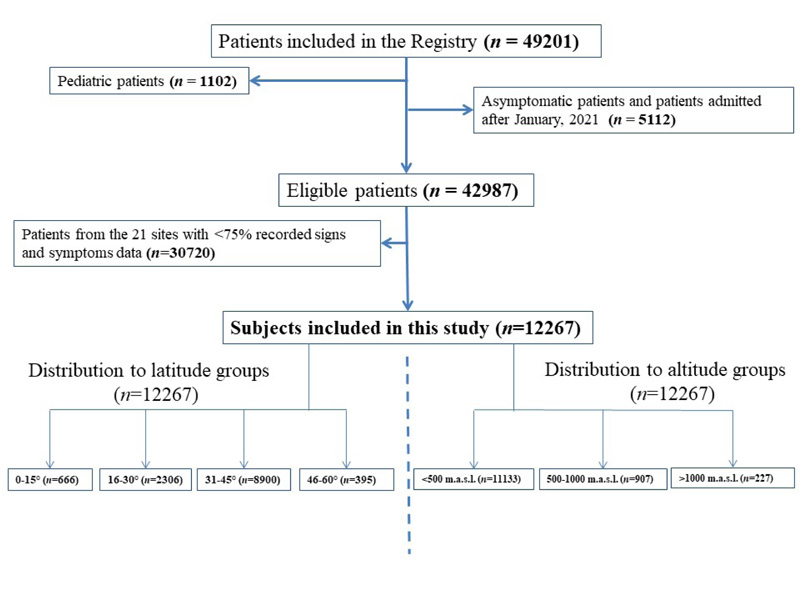
The most common symptom group was respiratory (N=9778, 80%), followed by constitutional (N=9216, 75%) and gastrointestinal (N=4351, 35%). Additionally, 12% of the patients presented with cardiovascular symptoms. The symptoms not listed in any essential references, i.e., atypical, were present in a considerable proportion of the patients (N=2603, 21%). The distribution of the symptoms according to latitude and altitude categories is summarized in Table 2.
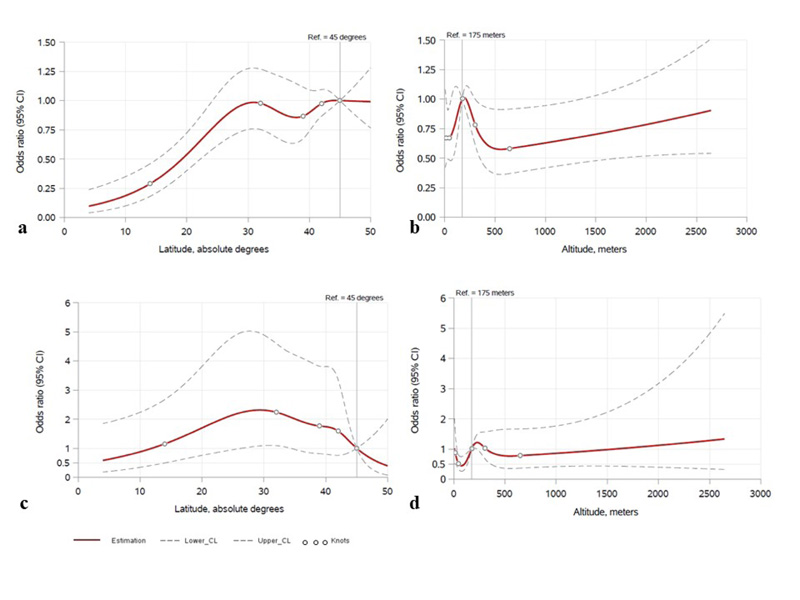
There was not enough evidence to suggest a relationship between latitude and odds of respiratory symptoms (adjusted odds ratio (OR) per 10° = 0.88, 95% confidence interval (CI) = 0.76, 1.00, p=.057). However, latitude was associated with cardiovascular symptoms, with higher latitude associated with higher odds of cardiovascular symptoms (adjusted OR = 1.22, 95%CI = 1.06, 1.42, p=.006). The data suggested a non-linear relationship between latitude and the presence of gastrointestinal symptoms, with lower odds of gastrointestinal symptoms in lower latitude locations (Fig. 2a), adjusted association p<.001, the odds ratios for 15º, 25º, and 30º latitude points were 0.32, 0.81, and 0.98, compared to the reference point of 45º). Specifically, the odds of gastrointestinal symptoms increased further away from the Equator up to the latitude of 30°. In unadjusted analyses, atypical symptoms were associated with latitude (p=.049), being more common between 20° and 40°. (Fig. 2b) however, this association did not remain significant after adjusting for potential confounders (p=.056) (Fig. 2c). There was little evidence to suggest a relationship between latitude and constitutional, neurological, or mucocutaneous symptoms (all p>0.05) (Table 3).
| Sign and symptom groups | Total (n=12267) | Latitude | Altitude | |||||
| 0-15°(n=666) | 16-30°(n=2306) | 31-45°(n=8900) | 46-60°(n=395) | <500 m.a.s.l. (n=11133) | 500 – 1000 m.a.s.l. (n=907) | >1000 m.a.s.l. (n=227) | ||
| Respiratory | 9778 (80%) | 580 (87%) | 1877 (81%) | 7011 (79%) | 310 (78%) | 8809 (79%) | 774 (85%) | 195 (86%) |
| Constitutional | 9216 (75%) | 550 (83%) | 1821 (79%) | 6528 (73%) | 317 (80%) | 8313 (75%) | 731 (81%) | 172 (76%) |
| Gastrointestinal | 4351 (35%) | 90 (14%) | 766 (33%) | 3348 (38%) | 147 (37%) | 4021 (36%) | 271 (30%) | 59 (26%) |
| Neurological | 3573 (29%) | 255 (38%) | 645 (28%) | 2541 (29%) | 132 (33%) | 3268 (29%) | 244 (27%) | 61 (27%) |
| Cardiovascular | 1522 (12%) | 76 (11%) | 279 (12%) | 1125 (13%) | 42 (11%) | 1398 (13%) | 96 (11%) | 28 (12%) |
| Mucocutaneous | 88 (1%) | 1 (0%) | 16 (1%) | 68 (1%) | 3 (1%) | 76 (1%) | 10 (1%) | 2 (1%) |
| Atypical | 2603 (21%) | 29 (4%) | 478 (21%) | 2006 (23%) | 90 (23%) | 2407 (22%) | 134 (15%) | 62 (27%) |
| Sign and symptom groups | Latitude | Altitude | |||||||||||
| Unadjusted | Adjusted | Unadjusted | Adjusted | ||||||||||
| Estimate* | 95% CI | p-value | Estimate* | 95% CI | p-value | Estimate* | 95% CI | p-value | Estimate* | 95% CI | p-value | ||
| Respiratory | 0.88 | (0.76, 1.01) | 0.074 | 0.88 | (0.76, 1.00) | 0.057 | 1.04 | (0.96, 1.13) | 0.326 | 1.04 | (0.96, 1.12) | 0.393 | |
| Constitutional | 1.00 | (0.86, 1.15) | 0.963 | 1.01 | (0.88, 1.16) | 0.877 | 0.98 | (0.94, 1.03) | 0.445 | 0.98 | (0.94, 1.02) | 0.289 | |
| Gastrointestinal | RCS, p-value non-linearity =0.0005, p-value overall association = <0.0001 | RCS, p-value non-linearity =0.0026, p-value overall association = <0.0001 | 0.96 | (0.90, 1.02) | 0.173 | 0.96 | (0.91, 1.02) | 0.154 | |||||
| Neurological | 1.06 | (0.92, 1.24) | 0.417 | 1.06 | (0.91, 1.23) | 0.449 | 0.99 | (0.93, 1.05) | 0.669 | 0.99 | (0.93, 1.05) | 0.706 | |
| Cardiovascular | 1.09 | (0.96, 1.25) | 0.199 | 1.22 | (1.06, 1.42) | 0.006 | RCS, p-value non-linearity = 0.122, p-value overall association = 0.193 | RCS, p-value non-linearity = 0.078, p-value overall association = 0.040 | |||||
| Mucocutaneous | 1.28 | (0.92, 1.78) | 0.145 | 1.31 | (0.93, 1.84) | 0.123 | 1.06 | (0.92, 1.22) | 0.453 | 1.05 | (0.91, 1.21) | 0.537 | |
| Atypical | RCS, p-value non-linearity = 0.030, p-value overall association= 0.049 | RCS, p-value non-linearity = 0.029, p-value overall association = 0.056 | RCS, p-value non-linearity = 0.344, p-value overall association = 0.287 | RCS, p-value non-linearity = 0.032, p-value overall association = 0.026 | |||||||||
For the latitude points of 15º, 25 º, and 30º, compared to the reference latitude of 45º; the odds ratios regarding gastrointestinal symptoms were 0.32, 0.81, and 0.98, whereas the odds ratios for the atypical symptoms were 1.23, 2.14, and 2.31, respectively. For the altitude points of 75 m.a.s.l., 125 m.a.s.l., 400 m.a.s.l., and 600 m.a.s.l., compared to the reference altitude of 175 m.a.s.l.; the odds ratios regarding cardiovascular symptoms were 0.71, 0.86, 0.63, and 0.58, whereas the odds ratios for the atypical symptoms were 0.44, 0.60, 0.84, and 0.77, respectively.
CI: confidence interval; m.a.s.l.: meters above sea level; RCS: restricted cubic spline.
While evaluating the impact of altitude on disease manifestations, there was no statistically significant difference between symptom groups other than those of the cardiovascular system and atypical symptoms (Table 3). After adjustment for age, sex, comorbidities, cardiovascular signs and atypical symptoms were found to be significantly associated with altitude (p=.040 and p=.026, respectively, the adjusted odds ratios for 75, 125, 400, and 600 m.a.s.l. altitude points were 0.71, 0.86, 0.63, and 0.58 for cardiovascular; and 0.44, 0.60, 0.84, and 0.77 for atypical symptoms, compared to the reference point of 175 m.a.s.l.) (Fig. 2b, 2d). Cardiovascular symptoms were more common in locations between 75 and 225 m.a.s.l.
4. DISCUSSION
We report the distribution of signs and symptoms according to different altitudes and latitudes within an international registry database of COVID-19 patients. We noted that the presentation of COVID-19 patients with gastrointestinal symptoms, such as nausea, vomiting, diarrhea, or abdominal pain, was significantly less frequent in locations closer to the Equator (<30º). Atypical symptoms, such as dysuria, or insomnia, were associated with altitude. Although there may be confounders related to temperature and humidity or country-specific factors such as cultural differences or local dietary habits, the differences may still be related to the impact of latitude and altitude on the disease process. We would like to add the caveat that the smaller sample sizes in certain latitude and altitude variations might have affected our results and to determine a true impact. Still, recognizing the variability of the symptoms in different locations might prove useful from a clinical standpoint.
In the studies conducted to determine the impact of climatological factors on COVID-19 cases and fatality rates as well as disease severity, it was suggested that disease dynamics were related to characteristics of the location [12-14, 30, 31]. The factors influencing these dynamics were suggested as Vitamin D, sunlight, temperature and humidity [32-36]. Also, a possible linkage to ethnicity, cytokines, and angiotensin-converting enzyme 2 (ACE-2) system was proposed [37]. Studies have suggested that altitude impacts case rates, supporting the ACE-2 network’s involvement [38]. However, the literature on its impact on fatality is not uniform [39]. Some other studies posited a relation to viral dynamics [12, 40]. Geographic factors were demonstrated to impact other respiratory infection processes for respiratory viruses, Mycobacterium tuberculosis, and other agents [41-43]. Considering the abovementioned literature, it appears intuitive that climatological conditions may influence COVID-19 symptoms, especially those of the respiratory system. Nonetheless, in our study, although respiratory symptoms showed a trend of increase while moving closer to the Equator and rising from the sea level, there was no statistically significant association.
Gastrointestinal tract symptomatology is one of the well-known presentations of the disease [44]. In our study, they were less common in places closer to the Equator, increasing until the latitude of 30° and essentially plateaued through the higher latitudes. Other studies have demonstrated that latitude is linked to some gastrointestinal system disorders, such as inflammatory bowel disease or malignancies of the gastrointestinal tract. Although the mechanism underlying this relationship has not been completely elucidated, temperature, sunlight exposure, or vitamin D levels were proposed as potential explanations [45, 46]. Also, it was suggested that socioeconomic status might impact gut immunobiology, by delaying or decreasing the exposure to infectious agents [47]. To the best of our knowledge, the relationship between gastrointestinal symptomatology of COVID-19 and latitude has not been investigated in detail yet. It has been postulated that gut microbiota might indirectly affect the interaction between the virus and the host cell by influencing the expression of ACE-2 receptors [48]. Interestingly, some evidence indicates that human gut microbial diversity may be related to differences in latitude [49, 50]. The expression of ACE-2 is known to serve as an entry point for severe acute respiratory syndrome coronavirus 2 [51, 52]. Although, at this point, it merely remains a hypothesis, latitude might influence the gastrointestinal flora, subsequently impacting the frequency of gastrointestinal symptoms of COVID-19 patients via the ACE-2 receptor pathway. Furthermore, although not reaching a significant level, we observed that the frequency of gastrointestinal symptoms decreased with increasing altitude. Most of the patients in our study sample located in the latitude levels between 16-45○ might impact our analyses. Thus, our results should be interpreted cautiously. Nevertheless, a lower threshold for COVID-19 testing might be suggested for patients presenting with gastrointestinal symptoms, especially in higher latitude settings.
Another noteworthy observation of this study was that the symptoms which we sorted as atypical (dysuria, insomnia, etc.) showed a significant association with altitude levels. They also tended to be more common in latitudes of 20 – 40°. Since all the common symptoms listed in the main references have been included in the data collection forms and grouped under one of the categories mentioned earlier, the remaining symptoms could be considered atypical symptoms for COVID-19 presentation. Since they are a largely heterogeneous group, the rationale behind this association is difficult to explain. Nevertheless, it highlights the need to be mindful of unusual presentations of COVID-19.
We also detected an association between altitude and frequency of cardiovascular symptoms, etc., chest pain and tightness. Severe acute respiratory syndrome coronavirus-2 infection was linked to cardiovascular complications [53, 54]. One mechanism underlying the cardiovascular injury in COVID-19 is the ACE-2 receptor-pathway [55-57]. As a physiological response to relative hypoxia, ACE-2 receptor levels tend to decrease, moving higher from the sea level [29]. This may explain the higher frequency of cardiovascular symptoms in our study population living at relatively lower altitudes. However, the overall association between altitude and cardiovascular symptomatology appears complex, with fluctuations across different altitude levels, as illustrated in Fig. (2b). Also, even though our results might suggest that different elevation levels affect disease presentation, not having enough altitude variation to test the effect of atmospheric oxygen pressure and the small sample size from extremes of altitudes make it hard to say with certainty what effect higher altitudes might have. Of note, 12.4% of the patients presented with chest pain and/or tightness, underscoring the importance of being cognizant of cardiovascular symptoms in the COVID-19 diagnosis.
This is the first study to report the association of latitude and altitude with symptoms among COVID-19 patients in a large, diverse sample. The global nature of the VIRUS: COVID-19 registry database permits the comparison of subjects from various climatological conditions. It also enhances the generalizability of the results. Furthermore, adjusting the findings according to the baseline characteristics such as age and comorbidities was also a considerable advantage.
The major drawback of our study is the lack of information regarding patients’ residential location or relevant travel information. Thus, the geographic location of the institutions had to be used as a surrogate for the area where the presenting symptoms developed. We do not have information about patient referrals from a far distance, so we cannot determine the patient’s exact location at the time of the disease. However, we believe the widespread travel restrictions might have offset this limitation during the study period. Furthermore, there was not a large sample of variety in either lower latitude or higher altitude settings reflecting the distribution of participating sites (and thus, patients) predominantly from North America. This might have influenced our results, which should be interpreted cautiously. Another limitation is that the project was conducted exclusively on hospitalized patients, encompassing a relatively severely affected patient population, which impedes the applicability of the results to all COVID-19 patients. Being a global study, local hospitalization criteria and the strain on the resources might impact our results. Additionally, to optimize the reliability of the frequency calculation, any institution that provided data on symptoms for less than 75% of its participants was excluded from the analysis. Another limitation was that comorbidity obesity was defined solely by the International Classification of Diseases-10 codes. It is known that the levels of body mass index statuses might impact the course of COVID-19. However, we did not include this in our analyses. Lastly, the patients’ symptoms may be prevalent due to the geographical prevalence rather than COVID-19.
CONCLUSION
Presenting symptoms of COVID-19 patients varied according to the altitude and latitude of care locations. Notably, respiratory symptoms were most reported but did not show an association with locations in this study. Presentation with gastrointestinal symptoms was less common in lower latitudes and atypical symptoms associated with altitude. Healthcare providers should be mindful of diverse symptomatology based on the climatological factors of the patient care locations.
AUTHORS' CONTRIBUTIONS
AT and RK prepared the first draft of this manuscript; SQ, RS, VB, MS, MB, and ND contributed to the design of the study and the data collection; AML, ACH, and PJS analyzed the data; DKS, RCC, NKJ, ABC, US, HLA, JLD, AKK, IBZ, ATL, MA, SKM, and KWD: contributed to data collection; LR and KB: helped with the data retrieval; VKK, JPD, AJW, OG, and SS: reviewed, edited, and provided critical feedback on the manuscript.
LIST OF ABBREVIATIONS
| ACE-2 | = Angiotensin-converting enzyme 2 |
| CI | = Confidence interval |
| COVID-19 | = Coronavirus disease 2019 |
| IQR | = Interquartile range |
| m.a.s.l. | = Meters above the sea-level |
| OR | = Odds ratio |
| SCCM | = Society of Critical Care Medicine |
| VIRUS | = Viral Infection and Respiratory Illness Universal Study |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The VIRUS: COVID-19 Registry global study was evaluated by the institutional review board at Mayo Clinic and approved as exempt (IRB:20-002610).
HUMAN AND ANIMAL RIGHTS
This study utilized no animals. All human research techniques followed the committee's ethical guidelines and the Helsinki Declaration of 1975.
CONSENT FOR PUBLICATION
Since the data was unidentified, the researchers could not seek consent for publication.
STANDARDS OF REPORTING
STROBE guidelines were followed for this study.
AVAILABILITY OF DATA AND MATERIALS
The data sets utilized in this investigation might be made available upon reasonable request to the authors [A.T] and [R.K].
FUNDING
The registry is partly funded by the Gordon and Betty Moore Foundation and Janssen Research & Development, LLC. They had no influence on the analysis, interpretation, and reporting of pooled data.
AJW receives funding from the National Institutes of Health/National Heart, Lung and Blood Institute grants R01HL151607, R01HL139751, R01HL136660, Agency of Healthcare Research and Quality, R01HS026485, Boston Biomedical Innovation Center/NIH/NHLBI 5U54HL119145-07 and royalties from UpToDate.
OG receives funding from the Agency of Healthcare Research and Quality R18HS 26609-2, National Institutes of Health/National Heart, Lung and Blood Institute: R01HL 130881, UG3/UH3HL 141722; Department of Defense DOD W81XWH; American Heart Association Rapid Response Grant – COVID-19; and royalties from Ambient Clinical Analytics. Inc.
R.K. receives funding from the National Institutes of Health/National Heart, Lung and Blood Institute: R01HL 130881, UG3/UH3HL 141722; Gordon and Betty Moore Foundation, and Janssen Research & Development, LLC; and royalties from Ambient Clinical Analytics. Inc. They had no influence on the acquisition, analysis, interpretation, and reporting of pooled data for this manuscript.
CONFLICT OF INTEREST
Dr. Salim Surani is the Co-Editor of the journal Open Respiratory Medicine Journal.
ACKNOWLEDGEMENTS
SCCM Discovery VIRUS investigators collaborative co-author list is provided in the supplementary material.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.


