All published articles of this journal are available on ScienceDirect.
Bronchoscopy Findings of Severe and Critical COVID-19 Patients Treated in ICU: A Year of Experience in a Developing Country
Abstract
Background:
Bronchoscopy procedure in patients with COVID-19 poses significant challenges, especially in a developing country with limited resources.
Objectives:
We aim to describe the clinical characteristics of severe and critical COVID-19 patients treated in an intensive care unit (ICU) and their bronchoscopy findings.
Methods:
We performed a retrospective analysis of clinical data of ICU patients with COVID-19 treated and received bronchoscopy procedures. This study retrospectively included all consecutive patients who underwent bronchoscopy at a teaching hospital in Depok, Indonesia, from May, 2020, until May, 2021.
Results:
A total of 57 bronchoscopy procedures in 54 patients were performed in this study. Primary procedure indications were retained mucus (68.4%) and ventilatory support weaning failure (15.8%). Bronchoscopic findings were mostly hyperaemic mucosa (95.00%) and purulent secretion (50.90%). Microbiological findings from bronchoalveolar samples were Acinetobacter baumanii, Klebsiella pneumoniae, and Candida albicans (33.3%, 26.6%, and 10.5%, respectively). The most common fungal isolated were Candida albicans (28%), followed by Candida tropicalis (16%) and Aspergillus sp. (8%). The overall length of hospital stay was 24 days, and the in-ICU stay was 22.06 ± 10.99 days. The patients’ survival of 28-days postprocedural outcome was 25.9% (14 subjects). Follow-up found that 20.4% of patients survived after sixty days of hospitalization.
Conclusion:
Diagnostic and therapeutic bronchoscopy in ICU patients with COVID-19 was safe and feasible to perform in developing countries with limited resources. It could help bronchial mucous clearance and confirm microbiological infection. The procedures should be strictly performed for patients with indications and comply with safety standards.
1. INTRODUCTION
The coronavirus disease 2019 (COVID-19) spreads widely and leads to high mortality. COVID-19 patients may quickly progress to acute respiratory distress syndrome (ARDS), requiring ICU [1]. In Indonesia, until January, 2021, the overall proportion of occupied ICU beds for COVID-19 was 62.8%. Jakarta region (83.6%) accounted for the highest proportion of occupied ICU beds [2]. Of COVID-19 patients with the critical condition in ICU, 45% received ventilator support. This worsening illness can lead to death [3]. COVID-19 patients admitted to the ICU may require prolonged treatment and tend to acquire bacterial and viral infections [4].
Bronchoscopy may be required in critically ill patients with COVID-19 to alleviate complications caused by the excess of bronchial mucociliary secretes, manage complications, and obtain samples for microbiological cultures to establish a definitive diagnosis [5]. Bronchoscopy is an essential diagnostic and therapeutic tool but an aerosol-generating procedure that is not routinely performed during the COVID-19 pandemic. Bronchoscopy remains one of the highest-risk procedures, and the consequences may be more severe for patients and healthcare providers. Nevertheless, the effectiveness and safety of COVID-19 patients in ICU who got a bronchoscopy procedure remain scared and have not been evaluated so far, particularly in developing countries. For this purpose, this research describes and characterizes bronchoscopy procedures performed on COVID-19 patients in ICU during the 12 months of the COVID-19 pandemic in developing countries at a teaching hospital in Depok, Indonesia, and discusses some safety concerns.
2. MATERIALS AND METHODS
2.1. Study Population
Patients who underwent bronchoscopy at the study site were consecutively enrolled between May, 2020, and May, 2021. The inclusion criteria were patients more than 18 years old diagnosed with SARS-CoV-2 positive by the qPCR examination during the hospital admission and admitted to ICU. The length of hospital stay was counted from hospital admission until the patient was discharged or died. The ICU stays measured from patients admitted to the ICU until discharged or died. The time from hospital admission to the ICU was also included in the collected data. Sixty days of survival were evaluated since the day of admission. Survival after the bronchoscopy procedure was evaluated until 28-days after the procedure.
2.2. Bronchoscopy Procedure
The medical team decided on the indication of the bronchoscopy, consisting of urgent life-saving intervention (hemoptysis, massive bleeding, foreign body aspiration), possible superinfection, airway management (retained mucus), weaning failure, and a combination of those [6]. The patients were sedated during the procedure using standard intravenous sedating agents (propofol, ketamine, and fentanyl). A fiber optic flexible bronchoscopy unit was used. The bronchoscopic examination included position checking of the orotracheal tube, direct inspection of the trachea and bronchial mucosa, suctioning of secretion, and insertion of mucoactive agents, if necessary, by using hypertonic saline combined with hyaluronic acid. The bronchoscopy findings evaluations were bronchial tree abnormalities (hyperemic, edematous, mucus plug, blood clot, and stenosis) and bronchial aspirate (purulent, bloody mucus, clear mucus, and thick mucous). Bronchoalveolar lavage (BAL) was collected using 60 mL saline for the bronchoscopic procedure, and a microbiological specimen was collected from the lower respiratory airways for a microbiological purpose [6, 7]. Bronchoscopy procedures were performed as quickly as possible, with the duration of each bronchoscopy being 30 minutes.
2.3. Safety Procedures
Trained pulmonologists and nurses performed all procedures at the bedside of the negatively pressured ICU cubicle. Level III Personal Protective Equipment (PPE) was used by all operators. Standard cleaning and disinfection were performed between procedures. Careful preparation and precautions are necessary as this procedure can lead to a pronounced formation of aerosols and can minimize the duration of the procedure. General anesthesia with a muscle relaxant is recommended in COVID-19 patients to reduce aerosol production. All equipment was prepared, including a bronchoscopy system (scope and screen), mucoactive drugs, and microbiological recipients. Flexible bronchoscopes were used in this study. The reprocessing flexible bronchoscope consists of precleaning, leakage testing, manual cleaning and rinsing, and disinfection and sterilization with high-level disinfection [8, 9].
2.4. Microbiological Evaluation
All biosafety requirements should be implemented during the storing and processing of the specimen. The BAL fluid retrieved was processed for aerobic, fungal, mycobacterial culture, and antimicrobial susceptibility tests. The BAL specimen was processed using standard protocols to detect the most common respiratory pathogens. BAL specimen was centrifuged, and then an aliquot of the pellet was streaked onto the surface of the same set of agar plate media (blood agar, chocolate agar, and MacConkey). The culture was incubated at 35-37°C for about 16-24 hours under aerobic conditions. At the end of the incubation, the plates were examined for the presence of significant pathogens. They were kept for further observation for up to 5 days if they were negative.
Other collected BAL aliquot was processed for fungal culture (incubated on Sabouraud Dextrose Agar under aerobic conditions at 35°C for seven days). Mycobacterium tuberculosis and other mycobacteria were detected by microscopic examination of Ziehl-Neelsen-stained smears cultured under aerobic conditions at 37°C. An antibiotic sensitivity test was done manually by the diffusion and dilution method. The result was interpreted as sensitive, intermediate, and resistant.
3. RESULTS
3.1. Study Population
We included 54 consecutive patients with characteristics that are described in Table 1. Most of the patients were 60 (21–79) years old males (61.1%). Almost all patients were in a critical degree (96.3%) and presented with chief complaints of shortness of breath (63.0%) with symptom onset of 3 (1–21) days. Most patients were hypertensive (68.6%) and in respiratory distress (88.9%) during the emergency ward admission. Hypertension (53.7%) and diabetes (25.9%) were commonly reported comorbidities. The most common initial radiographic finding during the emergency ward admission was a single abnormality (88.9%), typically pneumonia. Multiple radiographic abnormalities were presented in 5.6% of patients, consisting of pneumonia with a pulmonary mass, pulmonary atelectasis, and pleural effusion.
The patients stayed in the hospital for 24 (6–63) days with an in-ICU stay of 22.06±10.99 days and received ventilation support for 20 (0–63) days. Most of the patients were intubated and received mechanical ventilators (92.6%), among which four patients also received a tracheostomy. The patients received antiviral (66.7%), antibiotics (98.1%), and antifungal (38.9%) treatment before the bronchoscopy procedure. Most of the antivirals used were Remdesivir (35.2%), and at least two antiviral combinations were used for 7.4% of patients. Five patients (1.9%) received minimal single antibiotics, while 88.9% received two combination antibiotics therapy. Antifungal treatment was only given to 21 patients. Fluconazole was the most used antifungal (20.4%), followed by micafungin.
3.2. Bronchoscopy Procedure
Bronchoscopy was done 57 times among 54 patients. Three patients received more than one bronchoscopy procedure. The procedure was performed 13 (4-33) days after the emergency ward admission. The most common indications were retained mucus (68.4%), weaning failure (15.8%), and a combination of those (12.3%). The most frequent bronchoscopic findings were the hyperemic mucosa wall (93.0%), edematous mucosa wall (91.2%), mucus plug (42.1%), and blood clots (24.6%). As the bronchial hygiene/toilet procedure was performed, the most common bronchial aspirates were purulent mucus (20.9%), and blood in the mucus was retrieved from 13 patients. The average bronchial rinsing fluid was 35 mL. Representative images of bronchoscopic findings are presented in Fig. (1), and bronchoscopy findings are described in Table 2.
| Basic Characteristics | Results |
|---|---|
| Sex | - |
| Male (%) | 33 (61.1%) |
| Female (%) | 21 (38.9%) |
| Age, in years (median, range) | 60 (21 – 79) |
| BMI (median, range) | 25.98 (9.92 – 49.60) |
| At Emergency Unit Admission | Results |
| COVID-19 Clinical Grade | - |
| Severe (%) | 2 (3.7%) |
| Critical (%) | 52 (96.3%) |
| Chief Complaint | - |
| Shortness of Breath (%) | 34 (63.0%) |
| Cough (%) | 6 (11.1%) |
| Fever (%) | 5 (9.3%) |
| Fatigue (%) | 3 (5.6%) |
| Hemoptysis (%) | 2 (3.7%) |
| Impaired Consciousness (%) | 2 (3.7%) |
| Abdominal Pain (%) | 1 (1.9%) |
| Anosmia (%) | 1 (1.9%) |
| Symptom Onset in days (median, range) | 3 (1 – 21) |
| Clinical Fever (body temperature >37.3 °C) | - |
| Yes (%) | 10 (18.5%) |
| No (%) | 44 (81.5%) |
| Respiratory Distress (RR≥24/min or SpO2 ≤93%) | - |
| Yes (%) | 48 (88.9%) |
| No (%) | 6 (11.1%) |
| Comorbidities | - |
| Hypertension (%) | 29 (53.7%) |
| Diabetes (%) | 14 (25.9%) |
| Heart Failure (%) | 12 (22.2%) |
| Cardiovascular Disease (%) | 6 (11.1%) |
| Kidney Disease (%) | 2 (3.7%) |
| Comorbidities Present | - |
| None (%) | 19 (35.2%) |
| <2 (%) | 16 (29.6%) |
| ≥2 (%) | 19 (35.2%) |
| Radiographic Abnormality | - |
| None (%) | 3 (5.6%) |
| At least 1 Abnormality (%) | 48 (88.9%) |
| More than 1 Abnormality (%) | 3 (5.6%) |
| Time from Hospital Admission to Intensive Care Unit, in days (median, range) | 1 (0 – 19) |
| Length of Hospital Stay (Overall), in days (median, range) | 24 (6 – 63) |
| Intensive Care Stay, in days (mean ± SD) | 22.06 ± 10.99 |
| At the Intensive Care Unit | Results |
| Ventilation Support Duration, in days (median, range) | 20 (0 – 63) |
| Types of Ventilation Support | - |
| Mechanical ventilator (%) | 50 (92.6%) |
| High-flow nasal cannula (%) | 1 (1.9%) |
| Nasal cannula (%) | 3 (5.6%) |
| Types of Airway Support | - |
| Endotracheal tube (%) | 46 (85.2%) |
| Tracheostomy (%) | 4 (7.4%) |
| None (%) | 4 (7.4%) |
| BMI = Body Mass Index; RR = respiratory rate; SD = standard deviation. | |
| Bronchoscopy Procedure Timing from Admission, in days (median, range) | 13 (4 - 33) |
|---|---|
| Bronchoscopy Indication | Results |
| Retained mucus (%) | 39 (68.4%) |
| Ventilator weaning failure (%) | 9 (15.8%) |
| Combination of retained mucus and ventilator weaning failure (%) | 7 (12.3%) |
| Hemoptysis (%) | 2 (3.5%) |
| Bronchoscopy Finding | Results |
| Bronchial Tree Abnormalities | - |
| Hyperemic mucosa wall (%) | 53 (93.0%) |
| Edematous mucosa wall (%) | 52 (91.2%) |
| Mucus plug (%) | 24 (42.1%) |
| Blood clot (%) | 14 (24.6%) |
| Stenosis (%) | 6 (10.5%) |
| Bronchial Aspirate | - |
| Purulent (%) | 29 (50.9%) |
| Bloody mucus (%) | 13 (22.8%) |
| Clear mucus (%) | 8 (14.0%) |
| Thick mucus (%) | 7 (12.3%) |
3.3. BAL Culture
A total of 55 bacterial cultures, 50 fungal cultures, and 49 mycobacterium examinations were collected, as described in Table 3. Overall, 52 of the total 55 BAL samples contained bacteria. There were two patients’ samples that grew more than one bacterium. In one case, these were Enterococcus faecalis and Pseudomonas luteola, while the other case included Klebsiella oxycota and Acinetobacter baumannii. The most frequent bacteria were Acinetobacter baumannii (33.3%), Klebsiella pneumoniae (24.56%), and Klebsiella oxycota (10.53%). Enterococcus faecalis, Flavimonas (Pseudo) oryzihabitans, Klebsiella terrigena, and Weeksella virosa were detected in only one sample. There was 58.0% fungal growth, and the most fungal isolated were Candida albicans (28.0%), Candida tropicalis (16.0%), and Aspergillus sp. (8.0%). Two of the 49 BALs were positive for Mycobacterium detected by microscopic examination of Ziehl-Neelsen-stained smears.
As described in Fig. (2), the overall length of hospital stay was 24 days (range 6-63 days), and the in-ICU stay was 22.06±10.99 days. The patients’ survival of 28-days postprocedural outcome was 25.9% (14 subjects), while 74.1% of the patients died. Four patients did not improve even after 28 days of the bronchoscopy procedure.
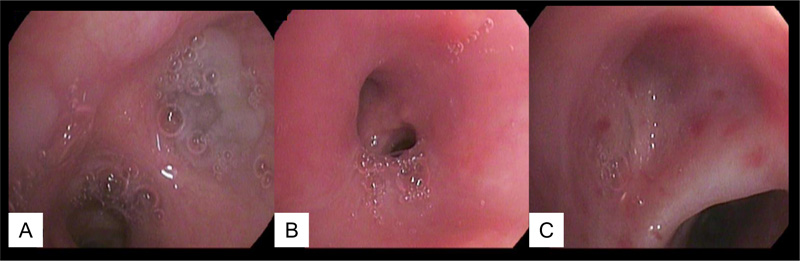
Bronchoscopic examination of the patient would generally reveal a) a mucous plug in at least one of the bronchial openings; b) an easy-to-bleed and hyperemic edematous mucosa along with thin mucous production; and c) a total closure of the small bronchial opening.
| Bacteria (n=55 samples) | Results |
|---|---|
| Acinetobacter baumannii | 19 (33.3%) |
| Klebsiella pneumoniae | 14 (24.56%) |
| Klebsiella oxycota | 6 (10.53%) |
| Escherichia coli | 5 (8.77%) |
| Pseudomonas luteola | 4 (7.02%) |
| Chryseomonas sp | 2 (3.51%) |
| Enterococcus faecalis | 1 (1.75%) |
| Flavimonas (Pseudo) oryzihabitans | 1 (1.75%) |
| Klebsiella terrigena | 1 (1.75%) |
| Weeksella virosa | 1 (1.75%) |
| No growth | 3 (5.26%) |
| Fungi (n=50 samples) | Results |
| Candida albicans | 14 (28.00%) |
| Candida tropicalis | 8 (16.00%) |
| Aspergillus sp | 4 (8.00%) |
| Candida glabrata | 1 (2.00%) |
| Candida krusei | 1 (2.00%) |
| Candida parapsilosis | 1 (2.00%) |
| No growth | 21 (42.00%) |
| Mycobacterium (n=49 samples) | Results |
| Positive | 2 (4.08%) |
| Negative | 47 (95.92%) |
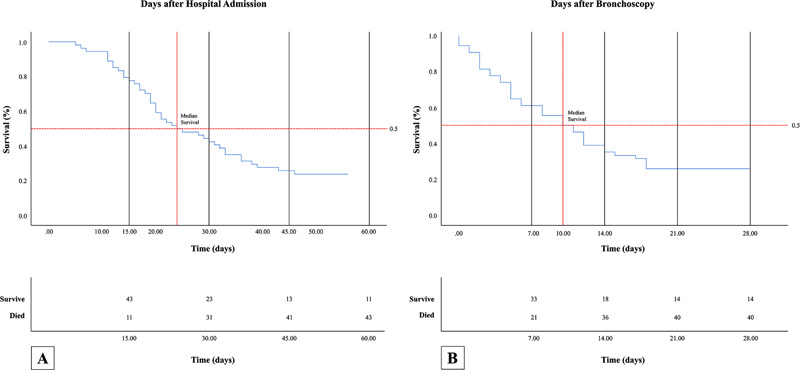
Survival analysis of patients in our study showed that: A) the overall median survival day after hospital admission was 24 days, while in-hospital survival rate at day 15, day 30, day 45, and day 60 were 43, 23, 13 and 11, respectively; B) the median survival day following bronchoscopy procedure was 10 days, while in-hospital survival rate at day-7, day-14, day-21 and day-28 were 33, 18, 14 and 14, respectively.
4. DISCUSSION
COVID-19 led to an increase in the number of critically ill patients. Accurate diagnosis of co-infection is vital for appropriate treatment since it has a worse outcome [10]. Bronchoscopy with BAL cultures is a helpful technique for diagnosing micro bacteria, which is expected to improve patient outcomes. Bronchoscopy procedures in severe COVID-19 patients are needed urgently for life saving and managing complications [6]. Bronchoscopy was selectively performed due to the concern of aerosolized infectious material [11]. Our study performed the bronchoscopy strictly for patients experiencing airway symptoms caused by thick mucus secretion and complicated ventilator weaning. In a case series from Surabaya, the bronchoscopic indication was worsening hypoxia, removing mucus plugs and microbiological sampling [12]. A study conducted in Spain reported that the primary indication of bronchoscopy in COVID-19 patients was improving mechanical ventilation (50%) and mucus plug/atelectasis (46%) [13]. According to the Joint Indian Chest Society, the common indications for diagnostic bronchoscopy were suspected infectious and for therapeutic bronchoscopy was the removal of retained secretion [14]. Bronchoscopy can be performed during the pandemic in some instances according to indications with the appropriate protocol [15].
In this study, the most common findings were hyperemic and edematous mucosa walls. The bronchial aspirate was mainly purulent infection (50.9%), which was consistent with findings of a study conducted by Bruyneel et al. (purulent plugs in 33 procedures) [16]. A study conducted by Torrego et al. also found that the most common complication in critically ill COVID-19 patients is thick hypersecretion in the airway (95%) [6]. An indication of mucus plugs was reported to increase the mortality rate by 60%, associated with worse outcomes than other indications [13]. Three intubated patients with COVID-19 showed improvement on chest X-Ray after bronchoscopy [12]. The bronchoscopy procedure can locate and allow the removal of a mucus plug which is one of the most frequent findings in COVID-19 patients in the ICU.
The bacteria detected from BAL samples in this study was 94.5%. Some studies found BAL culture-proven bacterial infection in 28.6% and 58.8% of cases [6, 16]. The Universitas Airlangga University Hospital in Surabaya, Indonesia, reported that sputum culture from COVID-19 patients with moderate to critical conditions was dominated by gram-negative bacteria (75.68%) [17]. A study at Dr. Soetomo Hospital, Surabaya, Indonesia, assessed that the most predominant isolates from sputum culture were Acinetobacter baumannii (38.0%) and Pseudomonas aeruginosa (22.0%) [18]. These studies used sputum samples with a higher probability of upper tract contamination than BAL samples from distal airways. In our study, Gram-negative bacteria, such as Acinetobacter baumannii and Klebsiella pneumoniae (33.3% and 24.6%), were the large majority of bacteria found in BAL culture. Several studies on bacterial infection of COVID-19 patients also showed similar results [19]. Sharifipour et al. reported Acinetobacter baumannii (17.9%) in adult patients admitted from the emergency ward to the ICU in Iran [20]. Other studies showed that the most common bacteria identified was Pseudomonas aeruginosa (84.6% and 38.8%, respectively) [6, 21]. Our study provides an idea about the actual conditions of bacterial infection in COVID-19 patients with severe conditions in the ICU of a referral hospital in Indonesia. Hence, it is necessary to pay attention to bacterial coinfection in critical patients with COVID-19 because it increases mortality rates [20].
COVID-19-associated pulmonary mycoses have been reported in multiple studies [22]. This study showed that 58% of COVID-19 patients in the ICU were found to have a fungal infection. Borg et al. reported fungal growth in BAL samples of COVID-19 patients in the ICU, including Aspergillus niger, Aspergillus flavus, and Candida glabrata [23]. Our study found that the most common fungal species were Candida albicans, Candida tropicalis, and Aspergillus sp. Candida sp. is an opportunistic pathogen commonly identified and remains a diagnostic dilemma as no test or threshold distinguishes contamination, commensalism, colonization, or etiologic in an infection [24]. Meanwhile, Koehler et al. suggested an increased risk of critically ill COVID-19 patients developing co-infection with Aspergillus, known as COVID-19-associated pulmonary aspergillosis (CAPA) [25]. CAPA is likely to increase the earlier onset of ICU admission and increase mortality [26]. We found 8% of CAPA from BAL culture. Routine testing for fungal infection in ICU patients with COVID-19 should be considered [25].
Most patients in this study were already receiving antibiotics (98.1%). Four patients received anti-tuberculosis drugs due to a positive mycobacterial result or the presence of relevant clinical features. Although Indonesia was endemic for tuberculosis and coinfection of COVID-19 with tuberculosis may result in a poorer outcome, the prevalence of tuberculosis was low in our study [27]. In consideration of the rapid spread of COVID-19 and the high burden of tuberculosis, especially in countries with high tuberculosis burden (India, China, and Indonesia, Filipina), routine screening for tuberculosis may be recommended among COVID-19 patients with the severe feature.
A study in Jakarta, Indonesia, reported that the overall length of hospital stay was 24 days (range, 13-36) [28], which was consistent with that of our study, i.e., 24 (range, 6–63) days. This finding was also similar to a study conducted in China that reported a total hospital stay (including ICU) of 24 days [29]. The length of hospital stay of COVID-19 patients in England was >16 days, a shorter length than our study. This gap may occur due to differences in the population and healthcare systems [30]. In-ICU stay among COVID-19 patients in this study was longer (22.06±10.99 days) compared to the study conducted in China, which reported 18 days (range, 11-33). Differences may occur due to the patient’s characteristics. In our study, the patients’ age was 60 years (range, 21-79) older than the patients included in a study conducted in China (56.7±15.4 days) [29]. The length of ICU stay in a study conducted in Iran was 15 days (2-39) [20]. A cohort study conducted in China showed that the in-ICU stay was eight days (range, 4-12) [31]. This wide gap may occur because no bacterial pathogen was found in patients on admission in their study, and the sample used was just 19 patients. It is known that the length of ICU stay can be prolonged if patients become coinfected, resulting in a higher cost of treatment and increased mortality [20]. Bronchoscopy may help to diagnose and resolve complications [13]. The role of bronchoscopy in COVID-19 patients is a matter of debate. The decision to perform or not perform bronchoscopy should be made after carefully weighing the potential benefits against the potential risks. Further investigation is therefore advisable to gain a better understanding of the bronchoscopy procedures in ICU patients with COVID-19.
In China, overall mortality varies from 3.1% to 61.5% [32]. The overall COVID-19 mortality in Jakarta, Indonesia, was 12%, lower than other reports involving a large cohort in high-income countries [28]. The ICU mortality rate of critically ill patients was 67% in the US and 42% in the UK study [33, 34]. Meanwhile, in China, it was more than 49% [35]. In our study, 43 (79.6%) patients died during ICU hospitalization. There are differences between Europe and Asia population demographics, the prevalence of comorbidities, and healthcare systems. Although this study does not describe the overall and ICU mortality rate in COVID-19 patients, it provides an overview of the mortality of COVID-19 patients in the ICU who underwent bronchoscopy.
Three patients underwent repeated bronchoscopy, an average of 17 days apart, with bronchial toilet indication in all procedures. Notable findings in repeated procedures were different bacterial growth in two patients. Overall, no immediate complications of bronchoscopy procedures occurred, but five patients died 24 hours after the procedure. The following analysis showed that 79.6% of patients died after sixty days of hospitalization. The patients’ survival of 28-days postprocedural outcome was 25.9%. Although most of our patients had poor outcomes, we cannot determine whether the bronchoscopy procedure's risk outweighs its benefit. The same concern was reported by a previous study, which highlighted the low number of subjects as a limitation [16]. Greater sample size should be considered to explore the risk and benefits of the procedure and its associated factors.
Bronchoscopy of COVID-19 patients in the ICU has been challenging. Our bronchoscopy procedure was designed to minimalize the risk of aerosolization of SARS-CoV-2. Rapid bronchoscopy was done with adequate precautions. Sedation using neuromuscular blockade was used to prevent aerosol-generating cough. The room was fully negative-pressured, and the personal safety measures during the procedure were carefully applied, complying with the standards of safety issued by the Indonesian Ministry of Health [36]. No healthcare provider from our hospital involved in the bronchoscopies became confirmed positive for COVID-19. Patient safety measures included monitoring vital signs, performing procedures as quickly as possible, and avoiding episodes of severe desaturation. Immediately after the procedure, the precleaning step was done to prevent drying secretions on the exterior and inner channels of the bronchoscope. Leakage tests aim to detect the exterior’s or interior’s physical breaks of the bronchoscope. The enzymatic detergent was used for manual cleaning, rinsing, and brushing steps. Furthermore, the last step is utilizing an automated washer machine with a cleaning cycle to provide additional cleaning. Bronchoscopy with adequate protection and infection control can be performed safely in all cases in which it is clinically indicated [37]. Our research proves that developing countries can carry out safe bronchoscopy for COVID-19 patients.
CONCLUSION
The one-year experience during the pandemic in our hospital showed that bronchoscopy in ICU patients with COVID-19 is safe. Bronchoscopy could help bronchial mucous clearance and confirm the suspicion of microbiological infection. Implementing bronchoscopy for COVID-19 patients in developing countries with limited resources is feasible but should be strictly performed for patients with indications and comply with safety standards.
LIST OF ABBREVIATIONS
| ARDS | = Acute Respiratory Distress Syndrome |
| BAL | = Bronchoalveolar Lavage |
| PPE | = Personal Protective Equipment |
| CAPA | = COVID-19 Associated Pulmonary Aspergillosis |
AUTHORS’ CONTRIBUTIONS
IPP, GP, HB, IM, and HAR contributed to the bronchoscopy procedure and data collection. RRDH, AR, DA, AK, and TK contributed to clinical data collection. IPP, RRDH, JZ, and TK contributed to statistical analysis. All authors contributed equally to the manuscript preparation.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Ethics Committee of the Faculty of Medicine, Universitas Indonesia - Dr. Cipto Mangunkusumo Hospital has authorized the approval of this study (Ethical approval number KET-299/UN2.F1/ETIK/PPM.00.02/202).
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. This research was conducted on humans in accordance with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS FOR REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the article are available within the article.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors would like to thank their families and all patients and their families, staff, managers, and hospital directors of the study site for their resilience during this difficult time.


